Clin Endosc.
2023 Jan;56(1):107-113. 10.5946/ce.2022.019.
A multicenter comparative study of endoscopic ultrasound-guided fine-needle biopsy using a Franseen needle versus conventional endoscopic ultrasound-guided fine-needle aspiration to evaluate microsatellite instability in patients with unresectable pancreatic cancer
- Affiliations
-
- 1Department of Gastroenterology, School of Medicine, Fukushima Medical University, Fukushima, Japan
- 2Department of Gastroenterology, Ohtanishinouchi Hospital, Koriyama, Japan
- 3Department of Gastroenterology, Aizu Medical Center, Fukushima Medical University, Aizuwakamatsu, Japan
- 4Department of Endoscopy, Fukushima Medical University Hospital, Fukushima, Japan
- 5Department of Diagnostic Pathology, School of Medicine, Fukushima Medical University, Fukushima, Japan
- 6Department of Hepato-Biliary-Pancreatic and Transplant Surgery, School of Medicine, Fukushima Medical University, Fukushima, Japan
- KMID: 2538758
- DOI: http://doi.org/10.5946/ce.2022.019
Abstract
- Background/Aims
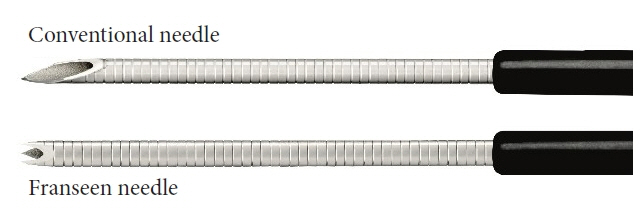
Immune checkpoint blockade has recently been reported to be effective in treating microsatellite instability (MSI)-high tumors. Therefore, sufficient sampling of histological specimens is necessary in cases of unresectable pancreatic cancer (UR-PC). This multicenter study investigated the efficacy of endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) using a Franseen needle for MSI evaluation in patients with UR-PC.
Methods
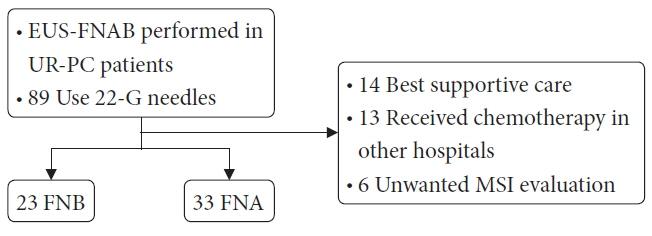
A total of 89 patients with UR-PC who underwent endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or EUS-FNB using 22-G needles at three hospitals in Japan (2018–2021) were enrolled. Fifty-six of these patients (FNB 23 and FNA 33) were followed up or evaluated for MSI. Patient characteristics, UR-PC data, and procedural outcomes were compared between patients who underwent EUS-FNB and those who underwent EUS-FNA.
Results
No significant difference in terms of sufficient tissue acquisition for histology was observed between patients who underwent EUS-FNB and those who underwent EUS-FNA. MSI evaluation was possible significantly more with tissue samples obtained using EUS-FNB than with tissue samples obtained using EUS-FNA (82.6% [19/23] vs. 45.5% [15/33], respectively; p<0.01). In the multivariate analysis, EUS-FNB was the only significant factor influencing the possibility of MSI evaluation.
Conclusions
EUS-FNB using a Franseen needle is desirable for ensuring sufficient tissue acquisition for MSI evaluation.
Keyword
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30.2. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010; 7:163–172.3. Warsame R, Grothey A. Treatment options for advanced pancreatic cancer: a review. Expert Rev Anticancer Ther. 2012; 12:1327–1336.4. Fogel EL, Shahda S, Sandrasegaran K, et al. A multidisciplinary approach to pancreas cancer in 2016: a review. Am J Gastroenterol. 2017; 112:537–554.5. Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020; 38:3217–3230.6. Okusaka T, Furuse J. Recent advances in chemotherapy for pancreatic cancer: evidence from Japan and recommendations in guidelines. J Gastroenterol. 2020; 55:369–382.7. Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999; 44:720–726.8. Yoshinaga S, Suzuki H, Oda I, et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011; 23 Suppl 1:29–33.9. Uehara H, Ikezawa K, Kawada N, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol. 2011; 26:1256–1261.10. Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel Franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019; 8:50–57.11. Sugiura R, Kuwatani M, Yane K, et al. Prospective, multicenter, observational study of tissue acquisition through EUS-guided fine-needle biopsy using a 25G Franseen needle. Endosc Ultrasound. 2019; 8:321–328.12. Sugimoto M, Irie H, Takagi T, et al. Efficacy of EUS-guided FNB using a Franseen needle for tissue acquisition and microsatellite instability evaluation in unresectable pancreatic lesions. BMC Cancer. 2020; 20:1094.13. Hikichi T, Irisawa A, Bhutani MS, et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009; 44:322–328.14. Suzuki R, Irisawa A, Bhutani MS, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012; 24:452–456.15. Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13:1035–1046.16. Japan Pancreas Society. General rules for the study of pancreatic cancer. 7th ed. Tokyo: Kanehara & Co., Ltd;2016.17. Yamamoto H, Itoh F, Nakamura H, et al. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001; 61:3139–3144.18. Murphy KM, Zhang S, Geiger T, et al. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn. 2006; 8:305–311.19. Kurata K, Kubo M, Kai M, et al. Microsatellite instability in Japanese female patients with triple-negative breast cancer. Breast Cancer. 2020; 27:490–498.20. Bando H, Okamoto W, Fukui T, et al. Utility of the quasi-monomorphic variation range in unresectable metastatic colorectal cancer patients. Cancer Sci. 2018; 109:3411–3415.21. Patil DT, Bronner MP, Portier BP, et al. A five-marker panel in a multiplex PCR accurately detects microsatellite instability-high colorectal tumors without control DNA. Diagn Mol Pathol. 2012; 21:127–133.22. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–458.23. Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy. 2017; 49:989–1006.24. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357:409–413.25. Luchini C, Grant RC, Scarpa A, et al. Microsatellite instability/mismatch repair deficiency in pancreatic cancers: the same or different? Gut. 2021; 70:1809–1811.26. Singhi AD, George B, Greenbowe JR, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology. 2019; 156:2242–2253.27. Kamatham S, Shahjehan F, Kasi PM. Circulating tumor DNA-based detection of microsatellite instability and response to immunotherapy in pancreatic cancer. Front Pharmacol. 2020; 11:23.28. Obayashi M, Shibasaki Y, Koakutsu T, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells curatively resected after pembrolizumab therapy for lung metastases: a case report. BMC Gastroenterol. 2020; 20:220.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors
- Role of Repeated Endoscopic Ultrasound-Guided Fine Needle Aspiration for Inconclusive Initial Cytology Result
- How Can We Get the Best Results with Endoscopic Ultrasound-Guided Fine Needle Aspiration?
- How to optimize the diagnostic yield of endoscopic ultrasoundguided fine-needle sampling in solid pancreatic lesions from a technical perspective