Lab Med Online.
2022 Oct;12(4):217-226. 10.47429/lmo.2022.12.4.217.
NUDT15 Genotyping in Thiopurine Drug Therapy
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea
- 2Department of Laboratory Medicine, Green Cross Laboratories, Yongin, Korea
- KMID: 2538609
- DOI: http://doi.org/10.47429/lmo.2022.12.4.217
Abstract
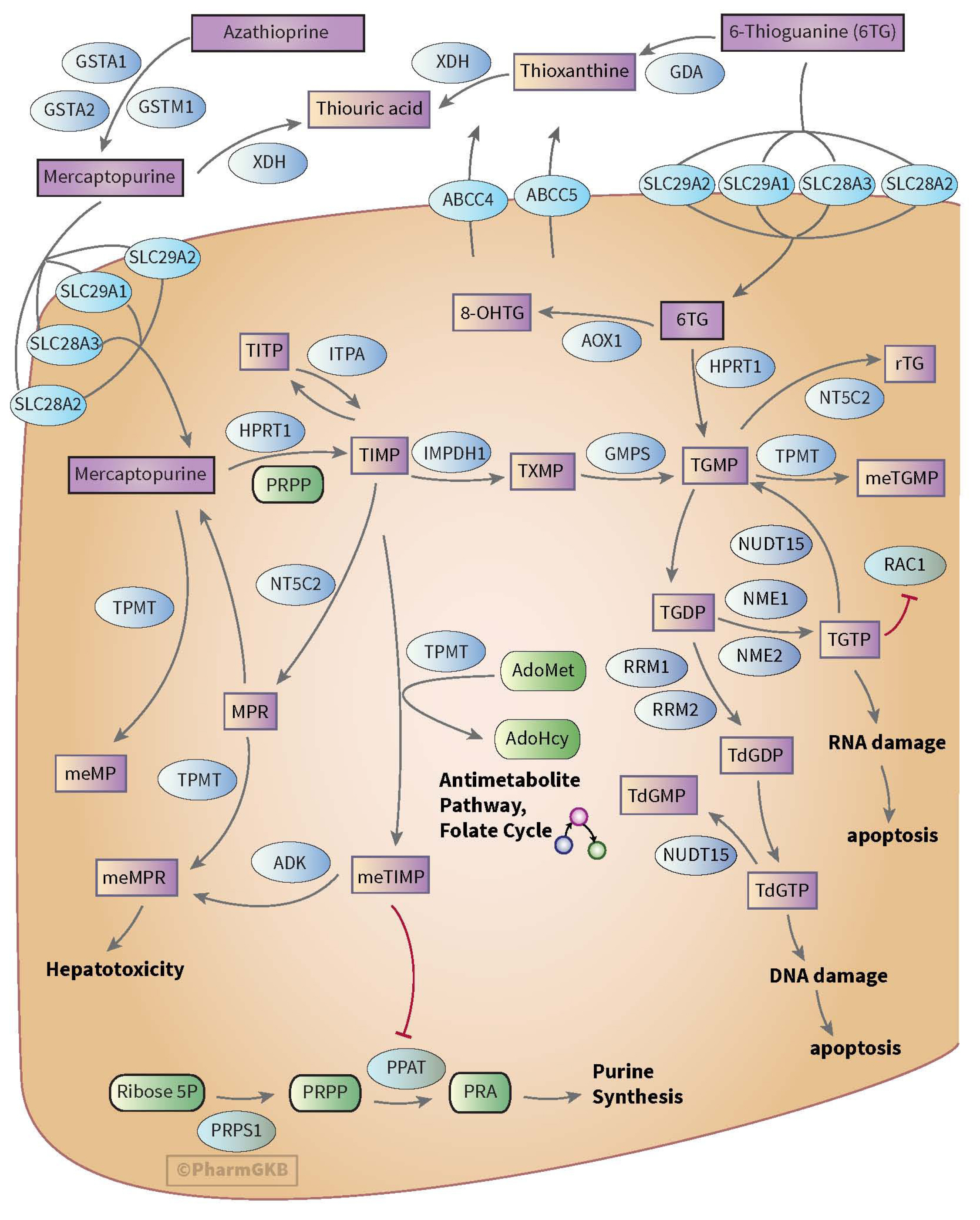
- Thiopurine is a pro-drug used to treat patients with inflammatory bowel disease, leukemia, malignancies, and autoimmune diseases as well as an immunosuppressive agent for post-transplantation states. Thiopurine drugs are metabolized into active metabolites by enzymes in various metabolic stages in the body. The importance of NUDT15 genotyping is emerging in the literature and clinical guidelines since it has been found to be associated with fatal thiopurine-related adverse drug reactions such as cytopenia, hepatotoxicity, and hair loss. Therefore, this review provides practical information about the clinical pharmacogenetic test for NUDT15 in patients treated with thiopurines to assist clinical laboratories in Korea. It focuses on thiopurine drug metabolism, clinical implications, and of NUDT15 genotyping. Moreover, it considers reports of pharmacogenetic test results, including current recommendations on NUDT15-guided thiopurine dosing.
Keyword
Figure
Reference
-
1. Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. 2019; Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin Pharmacol Ther. 105:1095–105. DOI: 10.1002/cpt.1304. PMID: 30447069. PMCID: PMC6576267.2. Moyer AM. 2021; NUDT15: A bench to bedside success story. Clin Biochem. 92:1–8. DOI: 10.1016/j.clinbiochem.2021.02.007. PMID: 33675810.3. Kim S, Yun YM, Chae HJ, Cho HJ, Ji M, Kim IS, et al. 2017; Clinical pharmacogenetic testing and application: Laboratory medicine clinical practice guidelines. Ann Lab Med. 37:180–93. DOI: 10.3343/alm.2017.37.2.180. PMID: 28029011. PMCID: PMC5204002.
Article4. Kim S, Yun YM, Kim IS, Song SH, Woo HI, Lee KA, et al. 2016; Clinical pharmacogenetic testing and application: laboratory medicine clinical practice guidelines part 2. Lab Med Online. 6:193–213. DOI: 10.3343/lmo.2016.6.4.193.
Article5. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. 2014; A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 46:1017–20. DOI: 10.1038/ng.3060. PMID: 25108385. PMCID: PMC4999337.
Article6. Kim HT, Choi R, Won HH, Choe YH, Kang B, Lee K, et al. 2017; NUDT15 genotype distributions in the Korean population. Pharmacogenet Genomics. 27:197–200. DOI: 10.1097/FPC.0000000000000274. PMID: 28277331.7. Valerie NC, Hagenkort A, Page BD, Masuyer G, Rehling D, Carter M, et al. 2016; NUDT15 hydrolyzes 6-thio-deoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 76:5501–11. DOI: 10.1158/0008-5472.CAN-16-0584. PMID: 27530327. PMCID: PMC6847052.
Article8. Evans WE. 2021; Improving the treatment of childhood acute lymphoblastic leukemia by optimizing the use of 70-year-old drugs. Haematologica. 106:2794–6. DOI: 10.3324/haematol.2021.278967. PMID: 34047180. PMCID: PMC8561287.
Article9. Larsen RH, Utke Rank C, Grell K, Nørgaard Møller L, Malthe Overgaard U, Kampmann P, et al. 2021; Increments in DNA-thioguanine level during thiopurine-enhanced maintenance therapy of acute lymphoblastic leukemia. Haematologica. 106:2824–33. DOI: 10.3324/haematol.2020.278166. PMID: 34047177. PMCID: PMC8561300.
Article10. Dean L, Kane M. Pratt VM, Scott SA, editors. Mercaptopurine Therapy and TPMT and NUDT15 genotype. Medical genetics summaries. https://www.ncbi.nlm.nih.gov/books/NBK100660/. Updated on Oct 2020.11. Dickson AL, Daniel LL, Zanussi J, Dale Plummer W, Wei WQ, Liu G, et al. 2022; TPMT and NUDT15 variants predict discontinuation of azathioprine for myelotoxicity in patients with iflammatory disease: real-world clinical results. Clin Pharmacol Ther. 111:263–71. DOI: 10.1002/cpt.2428. PMID: 34582038.12. Carreras-Puigvert J, Zitnik M, Jemth AS, Carter M, Unterlass JE, Hallström B, et al. 2017; A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat Commun. 8:1541. DOI: 10.1038/s41467-017-01642-w. PMID: 29142246. PMCID: PMC5688067.
Article13. Singh A, Mahajan R, Kedia S, Dutta AK, Anand A, Bernstein CN, et al. 2022; Use of thiopurines in iflammatory bowel disease: an update. Intest Res. 20:11–30. DOI: 10.5217/ir.2020.00155. PMID: 33845546. PMCID: PMC8831775.
Article14. American Gastroenterological Association. 2017; Therapeutic drug monitoring in iflammatory bowel disease: clinical decision support tool. Gastroenterology. 153:858–9. DOI: 10.1053/j.gastro.2017.07.039. PMID: 28778399.15. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. 2017; American gastroenterological association institute guideline on therapeutic drug monitoring in iflammatory bowel disease. Gastroenterology. 153:827–34. DOI: 10.1053/j.gastro.2017.07.032. PMID: 28780013.
Article16. Lee SD, Shivashankar R, Quirk D, Zhang H, Telliez JB, Andrews J, et al. 2021; Therapeutic drug monitoring for current and investigational iflammatory bowel disease treatments. J Clin Gastroenterol. 55:195–206. DOI: 10.1097/MCG.0000000000001396. PMID: 32740098. PMCID: PMC7960149.
Article17. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK. 2015; Use of Thiopurines in Iflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 13:193–207. DOI: 10.5217/ir.2015.13.3.193. PMID: 26130993. PMCID: PMC4479733.
Article18. Walker GJ, Harrison JW, Heap GA, Voskuil MD, Andersen V, Anderson CA, et al. 2019; Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with iflammatory bowel disease. JAMA. 321:773–85. DOI: 10.1001/jama.2019.0709. PMID: 30806694. PMCID: PMC6439872.19. Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, et al. 2021; Evidence-based clinical practice guidelines for iflammatory bowel disease 2020. J Gastroenterol. 56:489–526. DOI: 10.1007/s00535-021-01784-1. PMID: 33885977. PMCID: PMC8137635.
Article20. Lee YJ, Hwang EH, Park JH, Shin JH, Kang B, Kim SY. 2016; NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn's disease. Eur J Gastroenterol Hepatol. 28:475–8. DOI: 10.1097/MEG.0000000000000564. PMID: 26735160.21. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. 2019; British Society of Gastroenterology consensus guidelines on the management of iflammatory bowel disease in adults. Gut. 68:s1–106. DOI: 10.1136/gutjnl-2019-318484. PMID: 31562236. PMCID: PMC6872448.22. Choi R, Sohn I, Kim MJ, Woo HI, Lee JW, Ma Y, et al. 2019; Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 85:1585–97. DOI: 10.1111/bcp.13943. PMID: 30927276. PMCID: PMC6595296.
Article23. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. 2015; Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 33:1235–42. DOI: 10.1200/JCO.2014.59.4671. PMID: 25624441. PMCID: PMC4375304.24. Karol SE, Yang JJ. 2020; Pharmacogenomics and ALL treatment: How to optimize therapy. Semin Hematol. 57:130–6. DOI: 10.1053/j.seminhematol.2020.10.001. PMID: 33256902. PMCID: PMC7708673.
Article25. Khaeso K, Nakkam N, Komwilaisak P, Wongmast P, Chainansamit SO, Dornsena A, et al. 2021; Genetic polymorphisms of drug-metabolizing enzymes involved in 6-mercaptopurine-induced myelosuppression in Thai pediatric acute lymphoblastic leukemia patients. J Pediatr Genet. 10:29–34. DOI: 10.1055/s-0040-1715818. PMID: 33552635. PMCID: PMC7853920.
Article26. Fan POL, Leung KT, Chan KYY, Leung AWK, Lam GKS, Chow TTW, et al. 2022; ABCC4, ITPA, NUDT15, TPMT and their interaction as genetic predictors of 6-mercaptopurine intolerance in chinese patients with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 39:254–66. DOI: 10.1080/08880018.2021.1973628. PMID: 34665987.27. Ramalingam R, Kaur H, Scott JX, Sneha LM, Arun Kumar GP, inivasan A Sr, et al. 2021; Pharmacogenetic evaluation of 6-mercaptopurine-mediated toxicity in pediatric acute lymphoblastic leukemia patients from a South Indian population. Pharmacogenomics. 22:401–11. DOI: 10.2217/pgs-2020-0193. PMID: 33876659.
Article28. Tsujimoto S, Osumi T, Uchiyama M, Shirai R, Moriyama T, Nishii R, et al. 2018; Diplotype analysis of NUDT15 variants and 6-mercaptopurine sensitivity in pediatric lymphoid neoplasms. Leukemia. 32:2710–4. DOI: 10.1038/s41375-018-0190-1. PMID: 29967377. PMCID: PMC6289816.
Article29. Koutsilieri S, Caudle KE, Alzghari SK, Monte AA, Relling MV, Patrinos GP. 2019; Optimizing thiopurine dosing based on TPMT and NUDT15 genotypes: It takes two to tango. Am J Hematol. 94:737–40. DOI: 10.1002/ajh.25485. PMID: 30945335.30. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. 2016; NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 48:367–73. DOI: 10.1038/ng.3508. PMID: 26878724. PMCID: PMC5029084.
Article31. Tanaka Y, Saito Y. 2021; Importance of NUDT15 polymorphisms in thiopurine treatments. J Pers Med. 11:778. DOI: 10.3390/jpm11080778. PMID: 34442422. PMCID: PMC8399029.32. Kim SY, Shin JH, Park JS, Kang SY, Nam TS, Kim JK, et al. 2017; NUDT15 p.R139C variant is common and strongly associated with azathioprine-induced early leukopenia and severe alopecia in Korean patients with various neurological diseases. J Neurol Sci. 378:64–8. DOI: 10.1016/j.jns.2017.04.041. PMID: 28566182.33. Fan X, Yin D, Men R, Xu H, Yang L. 2019; NUDT15 polymorphism confer increased susceptibility to thiopurine-induced leukopenia in patients with autoimmune hepatitis and related cirrhosis. Front Pharmacol. 10:346. DOI: 10.3389/fphar.2019.00346. PMID: 31024313. PMCID: PMC6465603.34. Fei X, Shu Q, Zhu H, Hua B, Wang S, Guo L, et al. 2018; NUDT15 R139C variants increase the risk of azathioprine-induced leukopenia in Chinese autoimmune patients. Front Pharmacol. 9:460. DOI: 10.3389/fphar.2018.00460. PMID: 29867468. PMCID: PMC5949564.
Article35. Shih YC, Zou YR, Wang B, Zheng J, Pan M. 2019; Azathioprine-induced myelosuppression in two pemphigus vulgaris patients with homozygous polymorphism of NUDT15. J Dermatol. 46:e59–61. DOI: 10.1111/1346-8138.14542. PMID: 30035323.36. Saida K, Kamei K, Ogura M, Matsumura S, Kano Y, Sato M, et al. 2018; Azathioprine-induced agranulocytosis and severe alopecia after kidney transplantation associated with a NUDT15 polymorphism: A case report. Transplant Proc. 50:3925–7. DOI: 10.1016/j.transproceed.2018.04.039. PMID: 30577288.37. Yang JJ, Whirl-Carrillo M, Scott SA, Turner AJ, Schwab M, Tanaka Y, et al. 2019; Pharmacogene variation consortium gene introduction: NUDT15. Clin Pharmacol Ther. 105:1091–4. DOI: 10.1002/cpt.1268. PMID: 30515762. PMCID: PMC6465081.38. Wang Q, Mailloux J, Schwarz UI, Kim RB, Wilson A. 2022; A novel NUDT15 variant identified in Caucasian TPMT wild type patients with iflammatory bowel disease and azathioprine-related myelotoxicity. Pharmacogenet Genomics. 32:39–41. DOI: 10.1097/FPC.0000000000000449. PMID: 34751173.
Article39. Kim HY, Lee SH, Lee MN, Kim JW, Kim YH, Kim MJ, et al. 2015; Complete sequence-based screening of TPMT variants in the Korean population. Pharmacogenet Genomics. 25:143–6. DOI: 10.1097/FPC.0000000000000117. PMID: 25564374.40. Ran Z, Wu K, Matsuoka K, Jeen YT, Wei SC, Ahuja V, et al. 2021; Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of iflammatory bowel disease in Asia. J Gastroenterol Hepatol. 36:637–45. DOI: 10.1111/jgh.15185. PMID: 32672839.41. Agency. EM. https://ec.europa.eu/health/documents/community-register/2017/20170713138228/anx_138228_en.pdf2017.42. Buaboonnam J, ipatanatadasakul P Sr, Treesucon A, Glomglao W, Siraprapapat P, Narkbunnam N, et al. 2019; Effect of NUDT15 on incidence of neutropenia in children with acute lymphoblastic leukemia. Pediatr Int. 61:754–8. DOI: 10.1111/ped.13905. PMID: 31166660.43. Sutiman N, Chen S, Ling KL, Chuah SW, Leong WF, Nadiger V, et al. 2018; Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian iflammatory bowel disease patients. Pharmacogenomics. 19:31–43. DOI: 10.2217/pgs-2017-0147. PMID: 29210335. PMCID: PMC5753614.
Article44. Kojima Y, Hirotsu Y, Omata W, Sugimori M, Takaoka S, Ashizawa H, et al. 2018; Ifluence of NUDT15 variants on hematological pictures of patients with iflammatory bowel disease treated with thiopurines. World J Gastroenterol. 24:511–8. DOI: 10.3748/wjg.v24.i4.511. PMID: 29398872. PMCID: PMC5787786.45. Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. 2017; Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 19:215–23. DOI: 10.1038/gim.2016.87. PMID: 27441996. PMCID: PMC5253119.
Article46. Choi CH, Moon W, Kim YS, Kim ES, Lee BI, Jung Y, et al. 2017; Second Korean Guideline for the Management of Ulcerative Colitis. Korean J Gastroenterol. 69:1–28. DOI: 10.4166/kjg.2017.69.1.1. PMID: 28135789.
Article47. Choi R, Chun MR, Park J, Lee JW, Ju HY, Cho HW, et al. 2021; Quantification of thioguanine in DNA using liquid chromatography-tandem mass spectrometry for routine thiopurine drug monitoring in patients with pediatric acute lymphoblastic leukemia. Ann Lab Med. 41:145–54. DOI: 10.3343/alm.2021.41.2.145. PMID: 33063676. PMCID: PMC7591283.
Article48. Ju HY, Lee JW, Cho HW, Hyun JK, Ma Y, Yi ES, et al. 2021; DNA-thioguanine nucleotide as a treatment marker in acute lymphoblastic leukemia patients with NUDT15 variant genotypes. PLoS One. 16:e0245667. DOI: 10.1371/journal.pone.0245667. PMID: 33481917. PMCID: PMC7822258.49. Banerjee R, Ravikanth VV, Pal P, Bale G, Avanthi US, Goren I, et al. 2020; NUDT15 C415T variant compared with TPMT genotyping in predicting azathioprine-induced leucopenia: prospective analysis of 1014 iflammatory bowel disease patients in India. Aliment Pharmacol Ther. 52:1683–94.50. Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, et al. 2017; Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with iflammatory bowel disease: A multicenter analysis. Iflamm Bowel Dis. 23:1592–9. DOI: 10.1097/MIB.0000000000001148. PMID: 28570428.51. Wang HH, He Y, Wang HX, Liao CL, Peng Y, Tao LJ, et al. 2018; Comparison of TPMT and NUDT15 polymorphisms in Chinese patients with iflammatory bowel disease. World J Gastroenterol. 24:941–8. DOI: 10.3748/wjg.v24.i8.941. PMID: 29491687. PMCID: PMC5829157.52. Akiyama S, Matsuoka K, Fukuda K, Hamada S, Shimizu M, Nanki K, et al. 2019; Long-term effect of NUDT15 R139C on hematologic indices in iflammatory bowel disease patients treated with thiopurine. J Gastroenterol Hepatol. 34:1751–7. DOI: 10.1111/jgh.14693. PMID: 31045285.53. Sato T, Takagawa T, Kakuta Y, Nishio A, Kawai M, Kamikozuru K, et al. 2017; NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with iflammatory bowel diseases. Intest Res. 15:328–37. DOI: 10.5217/ir.2017.15.3.328. PMID: 28670229. PMCID: PMC5478757.
Article54. Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, et al. 2018; NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with iflammatory bowel disease: a multicenter study. J Gastroenterol. 53:1065–78. DOI: 10.1007/s00535-018-1486-7. PMID: 29923122. PMCID: PMC6132901.
Article55. Choi R, Lee MN, Kim K, Baek SY, Kim TJ, Hong SN, et al. 2020; Effects of various genetic polymorphisms on thiopurine treatment-associated outcomes for Korean patients with Crohn's disease. Br J Clin Pharmacol. 86:2302–13. DOI: 10.1111/bcp.14339. PMID: 32372428. PMCID: PMC7576620.
Article56. Puangpetch A, Tiyasirichokchai R, Pakakasama S, Wiwattanakul S, Anurathapan U, Hongeng S, et al. 2020; NUDT15 genetic variants are related to thiopurine-induced neutropenia in Thai children with acute lymphoblastic leukemia. Pharmacogenomics. 21:403–10. DOI: 10.2217/pgs-2019-0177. PMID: 32308129.
Article57. Yi ES, Choi YB, Choi R, Lee NH, Lee JW, Yoo KH, et al. 2018; NUDT15 variants cause hematopoietic toxicity with low 6-TGN levels in children with acute lymphoblastic leukemia. Cancer Res Treat. 50:872–82. DOI: 10.4143/crt.2017.283. PMID: 28903549. PMCID: PMC6056957.
Article58. Schaeffeler E, Jaeger SU, Klumpp V, Yang JJ, Igel S, Hinze L, et al. 2019; Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet Med. 21:2145–50. DOI: 10.1038/s41436-019-0448-7. PMID: 30728528. PMCID: PMC6752748.59. Whirl-Carrillo M, Huddart R, Gong L, Sangkuhl K, Thorn CF, Whaley R, et al. 2021; An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 110:563–72. DOI: 10.1002/cpt.2350. PMID: 34216021. PMCID: PMC8457105.
Article60. Gaedigk A, Casey ST, Whirl-Carrillo M, Miller NA, Klein TE. Pharmacogene Variation Consortium (PharmVar). https://www.pharmvar.org/about. Updated on Mar 2018.61. Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. 2010; Thiopurine pathway. Pharmacogenet Genomics. 20:573–4. DOI: 10.1097/FPC.0b013e328334338f. PMID: 19952870. PMCID: PMC3098750.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of thiopurine-induced early leukopenia in a Korean pediatric patient with Crohn’s disease who turned out to possess homozygous mutations in NUDT15 R139C
- NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease
- NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases
- Pharmacogenetics-based personalized treatment in patients with inflammatory bowel disease A review
- NUDT15 Variants Cause Hematopoietic Toxicity with Low 6-TGN Levels in Children with Acute Lymphoblastic Leukemia