Lab Med Online.
2022 Apr;12(2):100-108. 10.47429/lmo.2022.12.2.100.
HIV-1 Drug Resistance Mutations and Their Clinical Implications in South Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
- 2Division of Infectious Diseases, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea
- 3Department of Laboratory Medicine, Seoul National University Boramae Medical Center, Seoul, Korea
- 4Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2538592
- DOI: http://doi.org/10.47429/lmo.2022.12.2.100
Abstract
- Background
While the incidence of new human immunodeficiency virus (HIV) infections has decreased, the dramatic rise in antiretroviral therapy (ART) use will likely increase the prevalence of drug resistance mutations (DRMs). This study aimed to investigate the prevalence and profile of HIV-1 DRMs in ART-naïve and ART-experienced patients in South Korea and determine the correlation between the degree of DRM and the clinical response.
Methods
Thirty-six ART-naïve and 8 ART-experienced HIV-1–infected Korean patients referred for standard genotypic resistance testing (SGRT) between 2018 and 2019 were enrolled. Their SGRT results, viral loads, and CD4+ T cell counts were analyzed.
Results
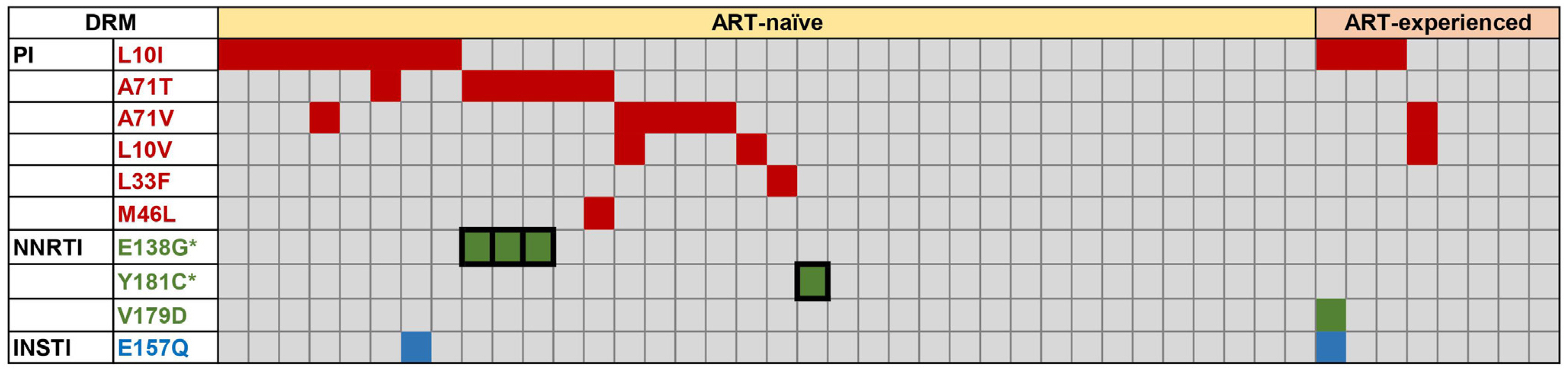
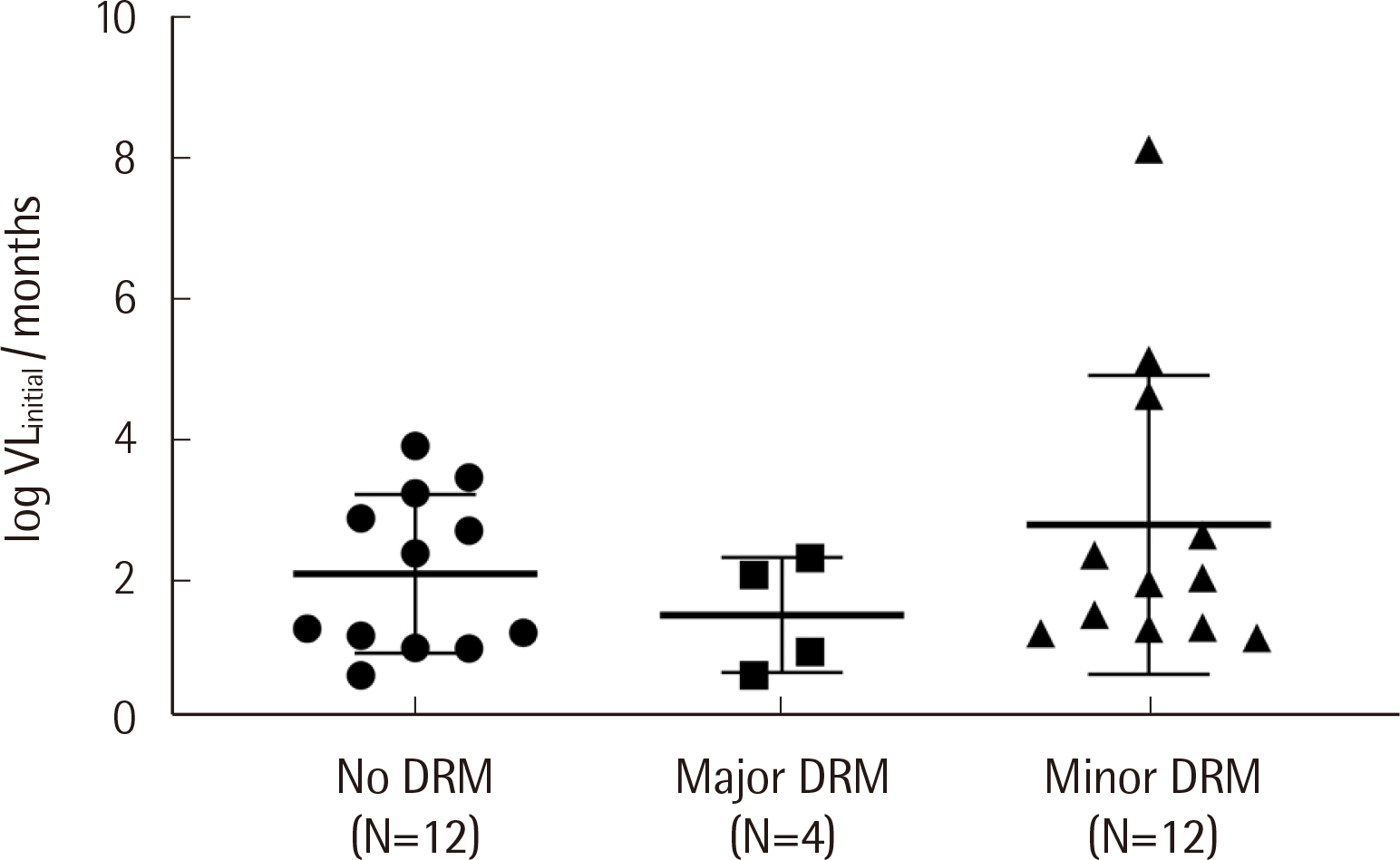
Protease inhibitor (PI)-related DRMs were the most frequently observed mutations in ART-naïve (52.8%) and ART-experienced (50.0%) groups, followed by nonnucleoside reverse transcriptase inhibitor (NNRTI)-related and integrase inhibitor (INSTI)-related DRMs. Major DRMs were observed only as NNRTI-related DRMs. The prevalence of transmitted drug resistance (TDR) was 55.6%, which was markedly higher than that previously reported. The changes in viral loads and CD4 counts in ART-naïve patients showed no correlation with the degree of DRM (major, minor, and none). All ART-naïve patients were treated with INSTI-based regimens, and most showed very good responses.
Conclusions
The distribution of HIV-1 DRMs in Korean patients was biased toward PI-related and minor DRMs, and DRM severity was not associated with the clinical response. This study provides valuable information on the recent DRM profile among Korean HIV-1 patients and emphasizes the importance of drug resistance genotyping.
Keyword
Figure
Reference
-
1. CDC NCHHSTP. New HIV Infections Drop 18 Percent in Six Years. https://www.hiv.gov/blog/new-hiv-infections-drop-18-percent-in-six-years. Updated on Feb 2017.2. Phillips AN, Cambiano V, Miners A, Revill P, Pillay D, Lundgren JD, et al. 2014; Effectiveness and cost-effectiveness of potential responses to future high levels of transmitted HIV drug resistance in antiretroviral drug-naive populations beginning treatment: modelling study and economic analysis. Lancet HIV. 1:e85–93. DOI: 10.1016/S2352-3018(14)70021-9. PMID: 26423990. PMCID: PMC4822192.
Article3. Panel on Antiretroviral Guidelines for Adults, Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Updated on Aug 2021.4. Hattori J, Shiino T, Gatanaga H, Mori H, Minami R, Uchida K, et al. 2016; Characteristics of transmitted drug-resistant HIV-1 in recently infected treatment-naive patients in Japan. J Acquir Immune Defic Syndr. 71:367–73. DOI: 10.1097/QAI.0000000000000861. PMID: 26428230.
Article5. Li T, Qian F, Yuan T, Xu W, Zhu L, Huang J, et al. 2017; Drug resistance mutation profiles of the drug-naïve and first-line regimen-treated HIV-1-infected population of Suzhou, China. Virol Sin. 32:271–9. DOI: 10.1007/s12250-017-4002-y. PMID: 28795354. PMCID: PMC6598913.
Article6. McColl DJ, Chen X. 2010; Strand transfer inhibitors of HIV-1 integrase: bringing IN a new era of antiretroviral therapy. Antiviral Res. 85:101–18. DOI: 10.1016/j.antiviral.2009.11.004. PMID: 19925830.
Article7. Meixenberger K, Yousef KP, Smith MR, Somogyi S, Fiedler S, Bartmeyer B, et al. 2017; Molecular evolution of HIV-1 integrase during the 20 years prior to the first approval of integrase inhibitors. Virol J. 14:223. DOI: 10.1186/s12985-017-0887-1. PMID: 29137637. PMCID: PMC5686839.8. Kim Y, Chin BS, Kim G, Shin HS. 2018; Integrase strand transfer inhibitor resistance mutations in antiretroviral treatment-naïve patients in Korea: a prospective, observational study. J Korean Med Sci. 33:e173. DOI: 10.3346/jkms.2018.33.e173. PMID: 29915524. PMCID: PMC6000596.
Article9. Onoriode Digban T, Chucks Iweriebor B, Chikwelu Obi L, Nwodo U, Ifeanyi Okoh A. 2020; Analyses of HIV-1 integrase gene sequences among treatment-naive patients in the Eastern Cape, South Africa. J Med Virol. 92:1165–72. DOI: 10.1002/jmv.25661. PMID: 31889319.
Article10. Park SW, Kim HB, Choi YJ, Kim NJ, Oh MD, Choe KW. 2003; Genotypic resistance of antiretroviral drugs among drug-naive HIV type 1 patients with the background of long-term access-easy zidovudine therapy. AIDS Res Hum Retroviruses. 19:1039–43. DOI: 10.1089/088922203322588404. PMID: 14686324.
Article11. Choi JY, Kim EJ, Park YK, Lee JS, Kim SS. 2008; National survey for drug-resistant variants in newly diagnosed antiretroviral drug-naive patients with HIV/AIDS in South Korea: 1999-2005. J Acquir Immune Defic Syndr. 49:237–42. DOI: 10.1097/QAI.0b013e318188a919. PMID: 18845957.
Article12. Bang JI, Song KH, Kim SH, Cho JH, Park WB, Park SW, et al. 2008; Prevalence of primary antiretroviral resistance: trends in Korea. AIDS Res Hum Retroviruses. 24:83–5. DOI: 10.1089/aid.2007.0116. PMID: 18275351.
Article13. Chin BS, Choi J, Nam JG, Kee MK, Suh SD, Choi JY, et al. 2006; Inverse relationship between viral load and genotypic resistance mutations in Korean patients with primary HIV type 1 infections. AIDS Res Hum Retroviruses. 22:1142–7. DOI: 10.1089/aid.2006.22.1142. PMID: 17147501.
Article14. Chin BS, Shin HS, Kim G, Wagner GA, Gianella S, Smith DM. 2015; Short Communication: Increase of HIV-1 K103N transmitted drug resistance and its association with efavirenz use in South Korea. AIDS Res Hum Retroviruses. 31:603–7. DOI: 10.1089/aid.2014.0368. PMID: 25826122. PMCID: PMC4516954.
Article15. Lwanga SK, Lemeshow S. Sample size determination in health studies: a practical manual. https://apps.who.int/iris/handle/10665/40062. Updated in 1991.16. Wensing AM, Calvez V, Ceccherini-Silberstein F, Charpentier C, Günthard HF, Paredes R, et al. 2019; 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med. 27:111–21. PMID: 31634862. PMCID: PMC6892618.17. Stanford HIVDB. Major HIV-1 Drug Resistance Mutations. https://cms.hivdb.org/prod/downloads/resistance-mutation-handout/resistance-mutation-handout.pdf. Updated on Oct 2020.18. Anstett K, Cutillas V, Fusco R, Mesplède T, Wainberg MA. 2016; Polymorphic substitution E157Q in HIV-1 integrase increases R263K-mediated dolutegravir resistance and decreases DNA binding activity. J Antimicrob Chemother. 71:2083–8. DOI: 10.1093/jac/dkw109. PMID: 27084918. PMCID: PMC4954922.
Article19. Kim MH, Song JE, Ahn JY, Kim YC, Oh DH, Choi H, et al. 2013; HIV antiretroviral resistance mutations among antiretroviral treatment-naive and -experienced patients in South Korea. AIDS Res Hum Retroviruses. 29:1617–20. DOI: 10.1089/aid.2013.0184. PMID: 23952717. PMCID: PMC3848436.
Article20. Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. 2009; Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 48:787–94. DOI: 10.1086/597093. PMID: 19193107. PMCID: PMC2720023.
Article21. Lok JJ, Bosch RJ, Benson CA, Collier AC, Robbins GK, Shafer RW, et al. 2010; Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS. 24:1867–76. DOI: 10.1097/QAD.0b013e32833adbcf. PMID: 20467286. PMCID: PMC3018341.
Article22. Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. 2001; Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 15:1369–77. DOI: 10.1097/00002030-200107270-00006. PMID: 11504958.
Article23. The Korean Society for AIDS. 2019; The 2018 Clinical Guidelines for the Diagnosis and Treatment of HIV/AIDS in HIV-Infected Koreans. Infect Chemother. 51:77–88. DOI: 10.3947/ic.2019.51.1.77. PMID: 30941943. PMCID: PMC6446007.24. Kim JY, Kim EJ, Choi JY, Kwon OK, Kim GJ, Choi SY, et al. 2011; Genetic variation of the HIV-1 integrase region in newly diagnosed anti-retroviral drug-naive patients with HIV/AIDS in Korea. Clin Microbiol Infect. 17:1155–9. DOI: 10.1111/j.1469-0691.2010.03392.x. PMID: 20946407.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Machine Learning to Improve the Effectiveness of ANRS in Predicting HIV Drug Resistance
- Integrase Strand Transfer Inhibitor Resistance Mutations in Antiretroviral Treatment-naïve Patients in Korea: a Prospective, Observational Study
- HIV drug resistance in treatment-naive HIV patients
- Prevalence of Zidovudine Resistant Human Immunodeficiency Virus Type 1 in Zidovudine-naive Patients
- Antiretroviral drug resistance among drug-naive HIV-1 infected patients