Cancer Res Treat.
2023 Jan;55(1):189-195. 10.4143/crt.2021.1527.
A Phase II Study of Preoperative Chemoradiotherapy with Capecitabine Plus Simvastatin in Patients with Locally Advanced Rectal Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2538004
- DOI: http://doi.org/10.4143/crt.2021.1527
Abstract
- Purpose
The purpose of this phase II trial was to evaluate whether the addition of simvastatin, a synthetic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, to preoperative chemoradiotherapy (CRT) with capecitabine confers a clinical benefit to patients with locally advanced rectal cancer (LARC).
Materials and Methods
Patients with LARC (defined by clinical stage T3/4 and/or lymph node positivity) received preoperative radiation (45-50.4 Gy in 25-28 daily fractions) with concomitant capecitabine (825 mg/m2 twice per day) and simvastatin (80 mg, daily). Curative surgery was planned 4-8 weeks after completion of the CRT regimen. The primary endpoint was pathologic complete response (pCR). The secondary endpoints included sphincter-sparing surgery, R0 resection, disease-free survival, overall survival, the pattern of failure, and toxicity.
Results
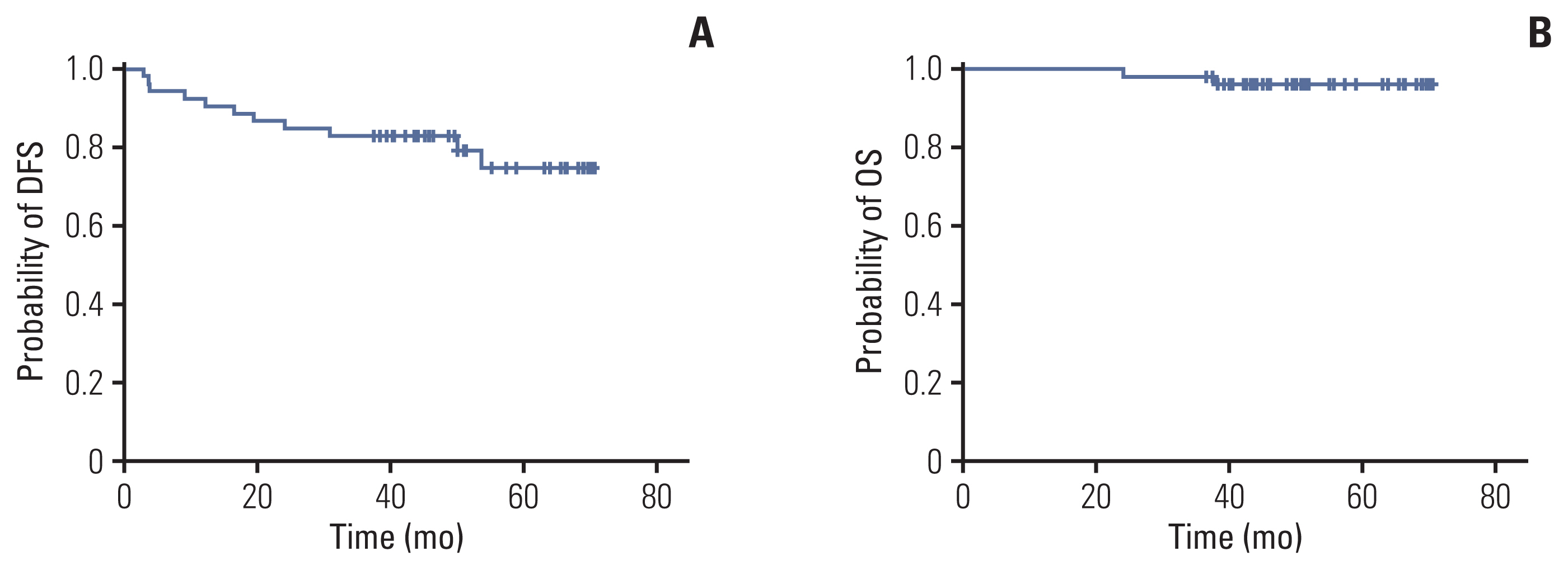
Between October 2014 and July 2017, 61 patients were enrolled; 53 patients completed CRT regimen and underwent total mesorectal excision. The pCR rate was 18.9% (n=10) by per-protocol analysis. Sphincter-sparing surgery was performed in 51 patients (96.2%). R0 resection was achieved in 51 patients (96.2%). One patient experienced grade 3 liver enzyme elevation. No patient experienced additional toxicity caused by simvastatin.
Conclusion
The combination of 80 mg simvastatin with CRT and capecitabine did not improve pCR in patients with LARC, although it did not increase toxicity.
Keyword
Figure
Reference
-
References
1. Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006; 24:4620–5.2. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006; 355:1114–23.3. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.4. O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994; 331:502–7.5. O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014; 32:1927–34.6. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012; 13:579–88.7. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014; 32:1554–62.8. Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011; 29:2773–80.9. Gerard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012; 30:4558–65.10. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990; 343:425–30.11. Casey PJ. Protein lipidation in cell signaling. Science. 1995; 268:221–5.12. Rando RR. Chemical biology of protein isoprenylation/methylation. Biochim Biophys Acta. 1996; 1300:5–16.13. Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006; 11:306–15.14. Lim T, Lee I, Kim J, Kang WK. Synergistic effect of simvastatin plus radiation in gastric cancer and colorectal cancer: implications of BIRC5 and connective tissue growth factor. Int J Radiat Oncol Biol Phys. 2015; 93:316–25.15. Sanli T, Liu C, Rashid A, Hopmans SN, Tsiani E, Schultz C, et al. Lovastatin sensitizes lung cancer cells to ionizing radiation: modulation of molecular pathways of radioresistance and tumor suppression. J Thorac Oncol. 2011; 6:439–50.16. Lacerda L, Reddy JP, Liu D, Larson R, Li L, Masuda H, et al. Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation. Stem Cells Transl Med. 2014; 3:849–56.17. Mace AG, Gantt GA, Skacel M, Pai R, Hammel JP, Kalady MF. Statin therapy is associated with improved pathologic response to neoadjuvant chemoradiation in rectal cancer. Dis Colon Rectum. 2013; 56:1217–27.18. Lee J, Jung KH, Park YS, Ahn JB, Shin SJ, Im SA, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol. 2009; 64:657–63.19. Lim SH, Kim TW, Hong YS, Han SW, Lee KH, Kang HJ, et al. A randomised, double-blind, placebo-controlled multi-centre phase III trial of XELIRI/FOLFIRI plus simvastatin for patients with metastatic colorectal cancer. Br J Cancer. 2015; 113:1421–6.20. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23.21. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–44.22. Gavioli M, Luppi G, Losi L, Bertolini F, Santantonio M, Falchi AM, et al. Incidence and clinical impact of sterilized disease and minimal residual disease after preoperative radiochemotherapy for rectal cancer. Dis Colon Rectum. 2005; 48:1851–7.23. Fokas E, Strobel P, Fietkau R, Ghadimi M, Liersch T, Grabenbauer GG, et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J Natl Cancer Inst. 2017; 109:djx095.24. Lee J, Hong YS, Hong JY, Han SW, Kim TW, Kang HJ, et al. Effect of simvastatin plus cetuximab/irinotecan for KRAS mutant colorectal cancer and predictive value of the RAS signature for treatment response to cetuximab. Invest New Drugs. 2014; 32:535–41.25. Longo J, van Leeuwen JE, Elbaz M, Branchard E, Penn LZ. Statins as anticancer agents in the era of precision medicine. Clin Cancer Res. 2020; 26:5791–800.26. Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JP 4th, et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2019; 176:564–80.27. Turrell FK, Kerr EM, Gao M, Thorpe H, Doherty GJ, Cridge J, et al. Lung tumors with distinct p53 mutations respond similarly to p53 targeted therapy but exhibit genotype-specific statin sensitivity. Genes Dev. 2017; 31:1339–53.28. Tutuska K, Parrilla-Monge L, Di Cesare E, Nemajerova A, Moll UM. Statin as anti-cancer therapy in autochthonous T-lymphomas expressing stabilized gain-of-function mutant p53 proteins. Cell Death Dis. 2020; 11:274.29. Lee J, Lee I, Han B, Park JO, Jang J, Park C, et al. Effect of simvastatin on cetuximab resistance in human colorectal cancer with KRAS mutations. J Natl Cancer Inst. 2011; 103:674–88.30. Nam GH, Kwon M, Jung H, Ko E, Kim SA, Choi Y, et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J Immunother Cancer. 2021; 9:e002474.31. Fritz G, Henninger C, Huelsenbeck J. Potential use of HMG-CoA reductase inhibitors (statins) as radioprotective agents. Br Med Bull. 2011; 97:17–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical issues in locally advanced rectal cancer treated by preoperative chemoradiotherapy
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer
- Prospective Phase II Study of Preoperative Chemoradiation with Capecitabine in Locally Advanced Rectal Cancer
- Long-term oncologic outcomes of neoadjuvant concurrent chemoradiotherapy with capecitabine and radical surgery in locally advanced rectal cancer: 10-year experiences at a single institution
- A Phase II Study of Additional Four-Week Chemotherapy With Capecitabine During the Resting Periods After Six-Week Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer