Korean J Pain.
2023 Jan;36(1):51-59. 10.3344/kjp.22297.
Imbalance in the spinal serotonergic pathway induces aggravation of mechanical allodynia and microglial activation in carrageenan inflammation
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- 2BioMedical Sciences Graduate Program (BMSGP), Chonnam National University Medical School, Hwasun, Korea
- KMID: 2537612
- DOI: http://doi.org/10.3344/kjp.22297
Abstract
- Background
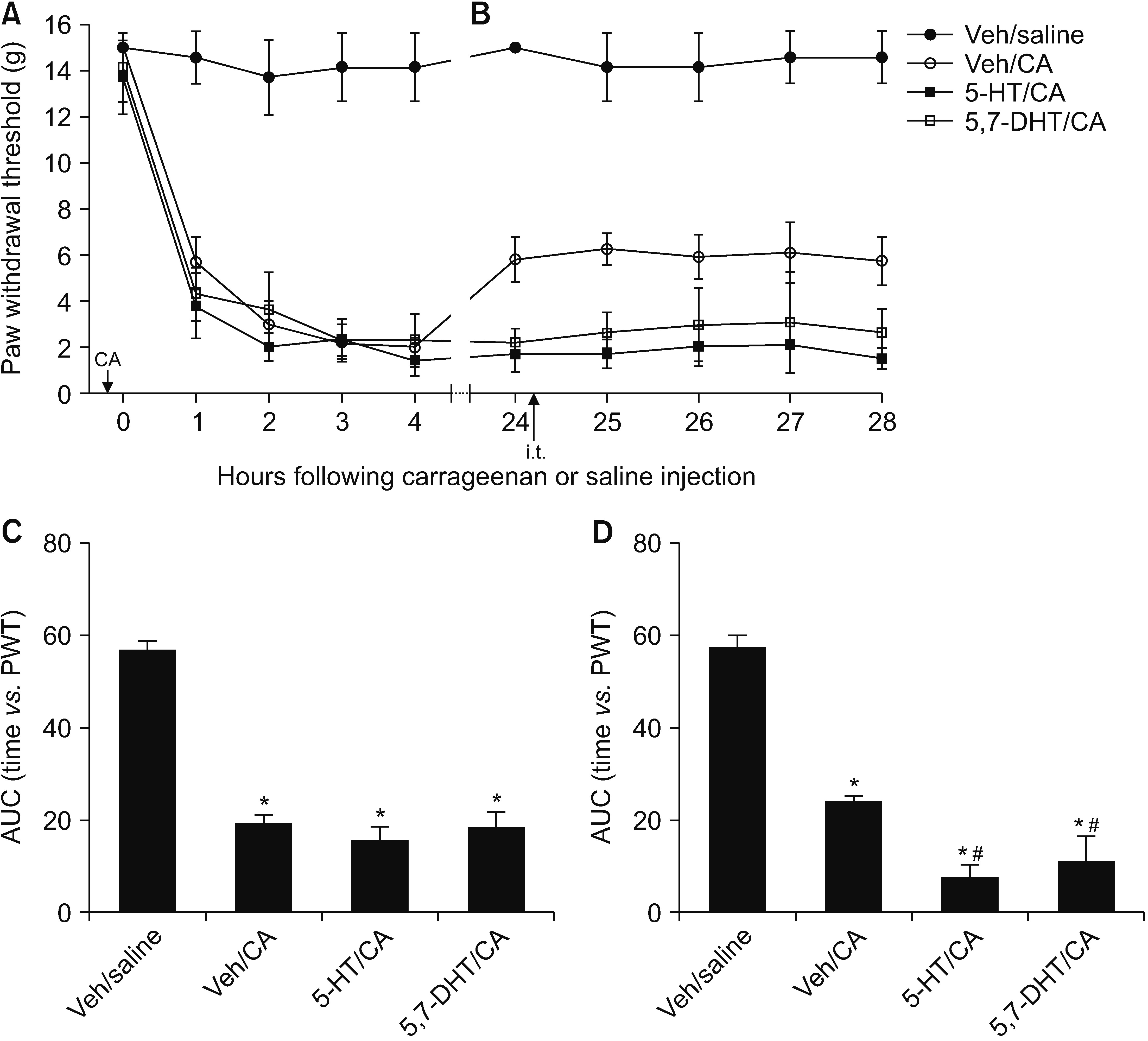
This study investigated the effect of an excess and a deficit of spinal 5-hydroxytryptamine (5-HT) on the mechanical allodynia and neuroglia activation in a rodent pain model of carrageenan inflammation.
Methods
Male Sprague–Dawley rats were implanted with an intrathecal (i.t.) catheter to administer the drug. To induce an excess or deficit of 5-HT in the spinal cord, animals were given either three i.t. 5-HT injections at 24-hour intervals or a single i.t. injection of 5,7-dihydroxytryptamine (5,7-DHT) before carrageenan inflammation. Mechanical allodynia was measured using the von Frey test for 0–4 hours (early phase) and 24–28 hours (late phase) after carrageenan injection. The changes in the activation of microglia and astrocyte were examined using immunofluorescence of the dorsal horn of the lumbar spinal cord.
Results
Both an excess and a deficit of spinal 5-HT had no or a minimal effect on the intensity of mechanical allodynia during the early phase but prevented the attenuation of mechanical allodynia during the late phase, which was observed in animals not treated with i.t. 5-HT or 5,7-DHT. Animals with an excess or deficit of 5-HT showed stronger activation of microglia, but not astrocyte, during the early and late phases, than did normal animals.
Conclusions
Imbalance in the descending 5-HT pathway in the spinal cord could aggravate the mechanical allodynia and enhance the activation of microglia, suggesting that the spinal 5-HT pathway plays an essential role in maintaining the nociceptive processing in balance between facilitation and inhibition in inflammatory pain caused by carrageenan inflammation.
Keyword
Figure
Reference
-
1. Basbaum AI, Bautista DM, Scherrer G, Julius D. 2009; Cellular and molecular mechanisms of pain. Cell. 139:267–84. DOI: 10.1016/j.cell.2009.09.028. PMID: 19837031. PMCID: PMC2852643.2. Donnelly CR, Andriessen AS, Chen G, Wang K, Jiang C, Maixner W, et al. 2020; Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics. 17:846–60. DOI: 10.1007/s13311-020-00905-7. PMID: 32820378. PMCID: PMC7609632.3. Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. 2013; Importance of glial activation in neuropathic pain. Eur J Pharmacol. 716:106–19. DOI: 10.1016/j.ejphar.2013.01.072. PMID: 23500198.4. Bardin L. 2011; The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 22:390–404. DOI: 10.1097/FBP.0b013e328349aae4. PMID: 21808193.5. Millan MJ. 2002; Descending control of pain. Prog Neurobiol. 66:355–474. DOI: 10.1016/S0301-0082(02)00009-6. PMID: 12034378.6. Sahbaie P, Irvine KA, Liang DY, Shi X, Clark JD. 2019; Mild traumatic brain injury causes nociceptive sensitization through spinal chemokine upregulation. Sci Rep. 9:19500. DOI: 10.1038/s41598-019-55739-x. PMID: 31863005. PMCID: PMC6925232.7. Guo W, Miyoshi K, Dubner R, Gu M, Li M, Liu J, et al. 2014; Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Mol Pain. 10:35. DOI: 10.1186/1744-8069-10-35. PMID: 24913307. PMCID: PMC4067691.8. Rahman W, Suzuki R, Webber M, Hunt SP, Dickenson AH. 2006; Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain. 123:264–74. DOI: 10.1016/j.pain.2006.02.033. PMID: 16644129.9. Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, et al. 2010; Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 30:8624–36. DOI: 10.1523/JNEUROSCI.5389-09.2010. PMID: 20573908. PMCID: PMC2902253.10. Yin M, Kim YO, Choi JI, Jeong S, Yang SH, Bae HB, et al. 2020; Antinociceptive role of neurotensin receptor 1 in rats with chemotherapy-induced peripheral neuropathy. Korean J Pain. 33:318–25. DOI: 10.3344/kjp.2020.33.4.318. PMID: 32989196. PMCID: PMC7532295.11. Deuis JR, Dvorakova LS, Vetter I. 2017; Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 10:284. DOI: 10.3389/fnmol.2017.00284. PMID: 28932184. PMCID: PMC5592204.12. Oatway MA, Chen Y, Weaver LC. 2004; The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 110:259–68. DOI: 10.1016/j.pain.2004.03.040. PMID: 15275776.13. Godínez-Chaparro B, López-Santillán FJ, Orduña P, Granados-Soto V. 2012; Secondary mechanical allodynia and hyperalgesia depend on descending facilitation mediated by spinal 5-HT₄, 5-HT₆ and 5-HT₇ receptors. Neuroscience. 222:379–91. DOI: 10.1016/j.neuroscience.2012.07.008. PMID: 22796074.14. Svensson CI, Tran TK, Fitzsimmons B, Yaksh TL, Hua XY. 2006; Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 580:6629–34. DOI: 10.1016/j.febslet.2006.11.012. PMID: 17113581. PMCID: PMC2291024.15. Yang J, Bae HB, Ki HG, Oh JM, Kim WM, Lee HG, et al. 2014; Different role of spinal 5-HT(hydroxytryptamine)7 receptors and descending serotonergic modulation in inflammatory pain induced in formalin and carrageenan rat models. Br J Anaesth. 113:138–47. Erratum in: Br J Anaesth 2015; 115: 154. DOI: 10.1093/bja/aet336. PMID: 24129596.16. Lee HG, Kim WM, Kim JM, Bae HB, Choi JI. 2015; Intrathecal nefopam-induced antinociception through activation of descending serotonergic projections involving spinal 5-HT7 but not 5-HT3 receptors. Neurosci Lett. 587:120–5. DOI: 10.1016/j.neulet.2014.12.040. PMID: 25534502.17. Kim JM, Jeong SW, Yang J, Lee SH, Kim WM, Jeong S, et al. 2015; Spinal 5-HT1A, not the 5-HT1B or 5-HT3 receptors, mediates descending serotonergic inhibition for late-phase mechanical allodynia of carrageenan-induced peripheral inflammation. Neurosci Lett. 600:91–7. DOI: 10.1016/j.neulet.2015.05.058. PMID: 26037417.18. Cragg JJ, Scott AL, Ramer MS. 2010; Depletion of spinal 5-HT accelerates mechanosensory recovery in the deafferented rat spinal cord. Exp Neurol. 222:277–84. DOI: 10.1016/j.expneurol.2010.01.005. PMID: 20079735.19. Géranton SM, Fratto V, Tochiki KK, Hunt SP. 2008; Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol Pain. 4:35. DOI: 10.1186/1744-8069-4-35. PMID: 18793388. PMCID: PMC2553762.20. Irvine KA, Sahbaie P, Ferguson AR, Clark JD. 2019; Enhanced descending pain facilitation in acute traumatic brain injury. Exp Neurol. 320:112976. DOI: 10.1016/j.expneurol.2019.112976. PMID: 31185197.21. Bradesi S. 2010; Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 22:499–511. DOI: 10.1111/j.1365-2982.2010.01491.x. PMID: 20236247. PMCID: PMC2893561.22. Rahman W, Suzuki R, Rygh LJ, Dickenson AH. 2004; Descending serotonergic facilitation mediated through rat spinal 5HT3 receptors is unaltered following carrageenan inflammation. Neurosci Lett. 361:229–31. DOI: 10.1016/j.neulet.2003.12.069. PMID: 15135935.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of the spinal 5-HT7 receptor and p-ERK pathway in the carrageenan inflammatory pain of rats
- Intrathecal Lamotrigine Attenuates Mechanical Allodynia and Suppresses Microglial and Astrocytic Activation in a Rat Model of Spinal Nerve Ligation
- Effect of Administering Freund's Complete Adjuvant to Spinal Nerves on the Development of Allodynia in the Rat
- Causalgiform pain produced by the tight ligation of L5, L6 spinal nerves in the rat
- Effect of Minocycline on Activation of Glia and Nuclear Factor kappa B in an Animal Nerve Injury Model