J Korean Med Sci.
2023 Jan;38(1):e9. 10.3346/jkms.2023.38.e9.
Vaccine Effect on Household Transmission of Omicron and Delta SARS-CoV-2 Variants
- Affiliations
-
- 1Division of Infectious Disease, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 2Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea
- 3Department of Statistics, Keimyung University, Daegu, Korea
- 4Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 5Department of Laboratory Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- KMID: 2537449
- DOI: http://doi.org/10.3346/jkms.2023.38.e9
Abstract
- Background
We evaluated the household secondary attack rate (SAR) of the omicron and delta severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, according to the vaccination status of the index case and household contacts; further, in vaccinated index cases, we evaluated the effect of the antibody levels on household transmission.
Methods
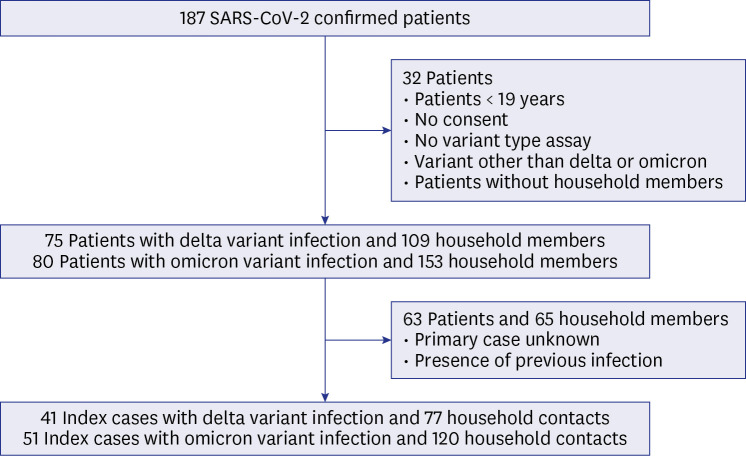
A prospective cross-sectional study of 92 index cases and 197 quarantined household contacts was performed. Tests for SARS-CoV-2 variant type and antibody level were conducted in index cases, and results of polymerase chain reaction tests (during the quarantine period) were collected from contacts. Association of antibody levels in vaccinated index cases and SAR was evaluated by multivariate regression analysis.
Results
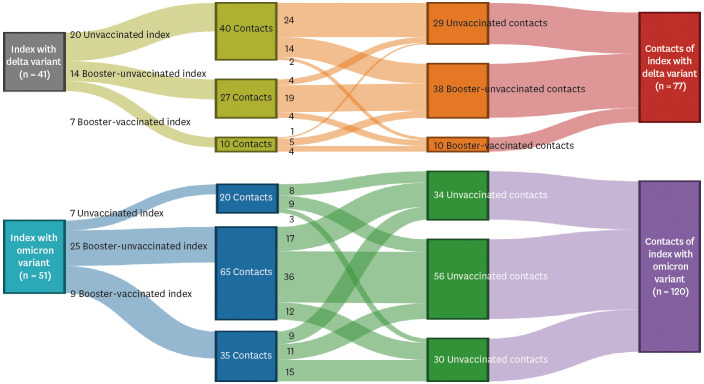
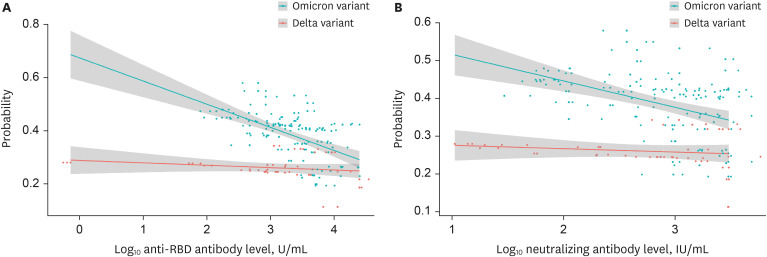
The SAR was higher in households exposed to omicron variant (42%) than in those exposed to delta variant (27%) (P = 0.040). SAR was 35% and 23% for unvaccinated and vaccinated delta variant exposed contacts, respectively. SAR was 44% and 41% for unvaccinated and vaccinated omicron exposed contacts, respectively. Booster dose immunisation of contacts or vaccination of index cases reduced SAR of vaccinated omicron variant exposed contacts. In a model with adjustment, anti-receptor-binding domain antibody levels in vaccinated index cases were inversely correlated with household transmission of both delta and omicron variants. Neutralising antibody levels had a similar relationship.
Conclusion
Immunisation of household members may help to mitigate the current pandemic.
Keyword
Figure
Reference
-
1. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.2. Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, et al. Effectiveness of COVID-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021; 385(15):1355–1371. PMID: 34496194.3. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021; 397(10287):1819–1829. PMID: 33964222.4. Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021; 373(1088):n1088. PMID: 33985964.5. Lee CM, Lee E, Park WB, Choe PG, Song KH, Kim ES, et al. Breakthrough COVID-19 infection during the delta variant dominant period: individualized care based on vaccination status is needed. J Korean Med Sci. 2022; 37(32):e252. PMID: 35971766.6. Layan M, Gilboa M, Gonen T, Goldenfeld M, Meltzer L, Andronico A, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. Am J Epidemiol. 2022; 191(7):1224–1234. PMID: 35238329.7. Prunas O, Warren JL, Crawford FW, Gazit S, Patalon T, Weinberger DM, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science. 2022; 375(6585):1151–1154. PMID: 35084937.8. Singanayagam A, Hakki S, Dunning J, Madon KJ, Crone MA, Koycheva A, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022; 22(2):183–195. PMID: 34756186.9. Eyre DW, Taylor D, Purver M, Chapman D, Fowler T, Pouwels KB, et al. Effect of COVID-19 vaccination on transmission of alpha and delta variants. N Engl J Med. 2022; 386(8):744–756. PMID: 34986294.10. Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021; 27(5):790–792. PMID: 33782619.11. World Health Organization. WHO coronavirus (COVID-19) dashboard. Updated 2022. Accessed February 11, 2022. https://covid19.who.int/ .12. Kim MK, Lee B, Choi YY, Um J, Lee KS, Sung HK, et al. Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea. J Korean Med Sci. 2022; 37(3):e31. PMID: 35040299.13. Lee JJ, Choe YJ, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (omicron) variant of concern in Korea, November 2021. J Korean Med Sci. 2021; 36(50):e346. PMID: 34962117.14. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021; 385(24):e85. PMID: 34706170.15. Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2022; 602(7898):676–681. PMID: 35016198.16. Kim MH, Nam Y, Son NH, Heo N, Kim B, Kang E, et al. antibody level predicts the clinical course of breakthrough infection of COVID-19 caused by delta and omicron variants: a prospective cross-sectional study. Open Forum Infect Dis. 2022; 9(7):ofac262. PMID: 35855960.17. He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020; 26(5):672–675. PMID: 32296168.18. Jørgensen SB, Nygård K, Kacelnik O, Telle K. Secondary attack rates for omicron and delta variants of SARS-CoV-2 in Norwegian households. JAMA. 2022; 327(16):1610–1611. PMID: 35254379.19. Del Águila-Mejía J, Wallmann R, Calvo-Montes J, Rodríguez-Lozano J, Valle-Madrazo T, Aginagalde-Llorente A. Secondary attack rate, transmission and incubation periods, and serial interval of SARS-CoV-2 omicron variant, Spain. Emerg Infect Dis. 2022; 28(6):1224–1228. PMID: 35393009.20. Lyngse FP, Mortensen LH, Denwood MJ, Christiansen LE, Møller CH, Skov RL, et al. Household transmission of the SARS-CoV-2 omicron variant in Denmark. Nat Commun. 2022; 13(1):5573. PMID: 36151099.21. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open. 2022; 5(4):e229317. PMID: 35482308.22. Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. The omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022; 185(3):447–456.e11. PMID: 35026151.23. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID: 35249272.24. Kodera S, Rashed EA, Hirata A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan. Vaccines (Basel). 2022; 10(3):430. PMID: 35335062.25. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022; 386(19):1804–1816. PMID: 35263534.26. Stokel-Walker C. What do we know about COVID vaccines and preventing transmission? BMJ. 2022; 376:o298. PMID: 35121611.27. Baker JM, Nakayama JY, O’Hegarty M, McGowan A, Teran RA, Bart SM, et al. SARS-CoV-2 B.1.1.529 (omicron) variant transmission within households - four U.S. jurisdictions, November 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(9):341–346. PMID: 35238860.28. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell. 2022; 185(3):457–466.e4. PMID: 34995482.29. Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022; 375(6581):678–680. PMID: 35040667.30. Central Disaster Management Headquarters (KR). Establishment of quarantine and in-home treatment system considering omicron characteristics. Updated 2022. Accessed March 7, 2022. http://ncov.mohw.go.kr/tcmBoardView.do?brdId=&brdGubun=&dataGubun=&ncvContSeq=370082&contSeq=370082&board_id=&gubun=ALL .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea
- mRNA vaccine effectiveness against SARS-CoV-2 B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant transmission from home care cases to household contacts in South Korea
- Recombinant proteins of spike protein of SARS-CoV-2 with the Omicron receptor-binding domain induce production of highly Omicron-specific neutralizing antibodies
- SARS-CoV-2 vaccine challenge based on spike glycoprotein against several new variants
- SARS-CoV-2 Omicron Variant of Concern: Everything You Wanted to Know about Omicron but Were Afraid to Ask