Ann Surg Treat Res.
2022 Dec;103(6):313-322. 10.4174/astr.2022.103.6.313.
Omission of chemotherapy for hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: patterns of treatment and outcomes from the Korean Breast Cancer Society Registry
- Affiliations
-
- 1Department of Surgery, Chong Hua Hospital-Cebu, Cebu City, Philippines
- 2Department of Pathology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 3Division of Breast and Endocrine Surgery, Department of Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 4Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea
- 5Department of Surgery, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- 6Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Surgery, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea
- 8Department of Surgery and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 9Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2536907
- DOI: http://doi.org/10.4174/astr.2022.103.6.313
Abstract
- Purpose
Although adjuvant chemotherapy (CTx) is still recommended for high-risk patients with hormone receptorpositive and human epidermal receptor (HER)-2-negative breast cancer, recent studies found that selected patients with low disease burden may be spared from CTx and receive hormonal treatment (HT) alone. This study aims to evaluate the trends of treatment (CTx + HT vs. HT alone) in Korea and to assess the impact on overall survival (OS) according to treatment pattern.
Methods
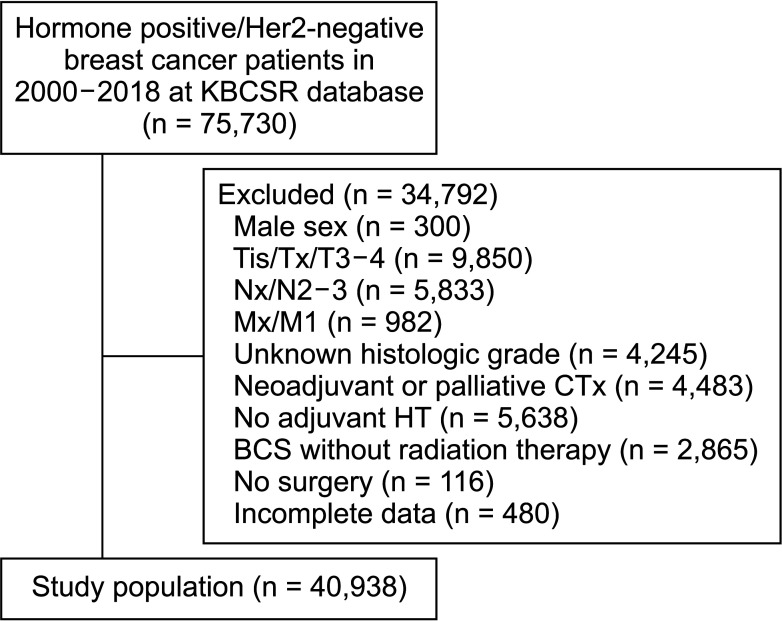
The Korean Breast Cancer Society Registry was queried (2000 to 2018) for women with pT1-2N0-1 hormone receptor-positive and HER2-negative disease who underwent surgery and adjuvant systemic treatment (CTx and HT). Clinicopathologic factors, change in pattern of treatment over time, and OS for each treatment option were analyzed.
Results
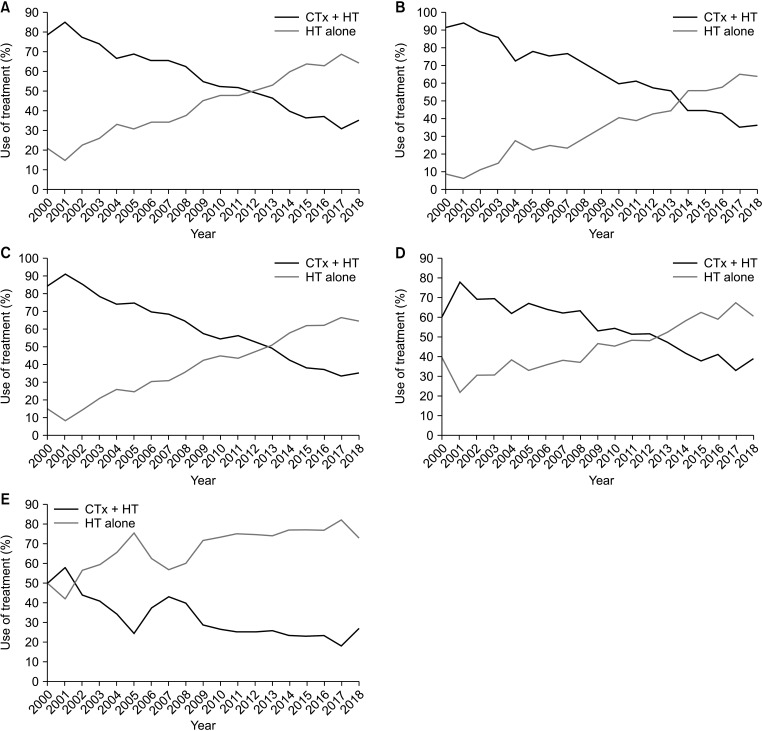
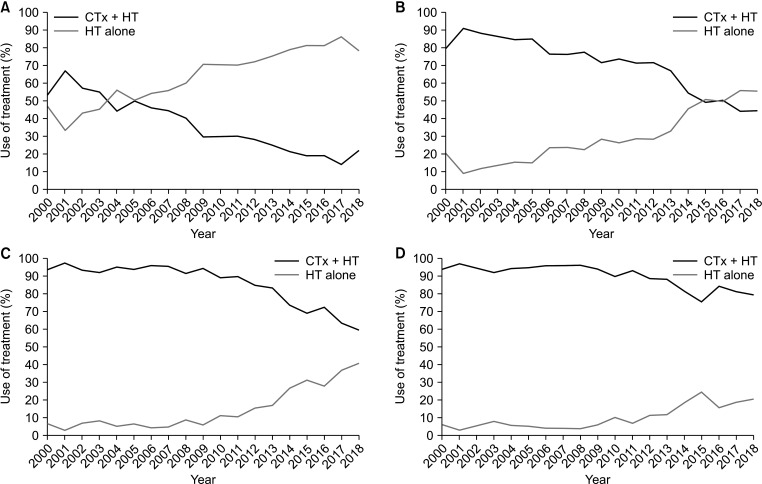
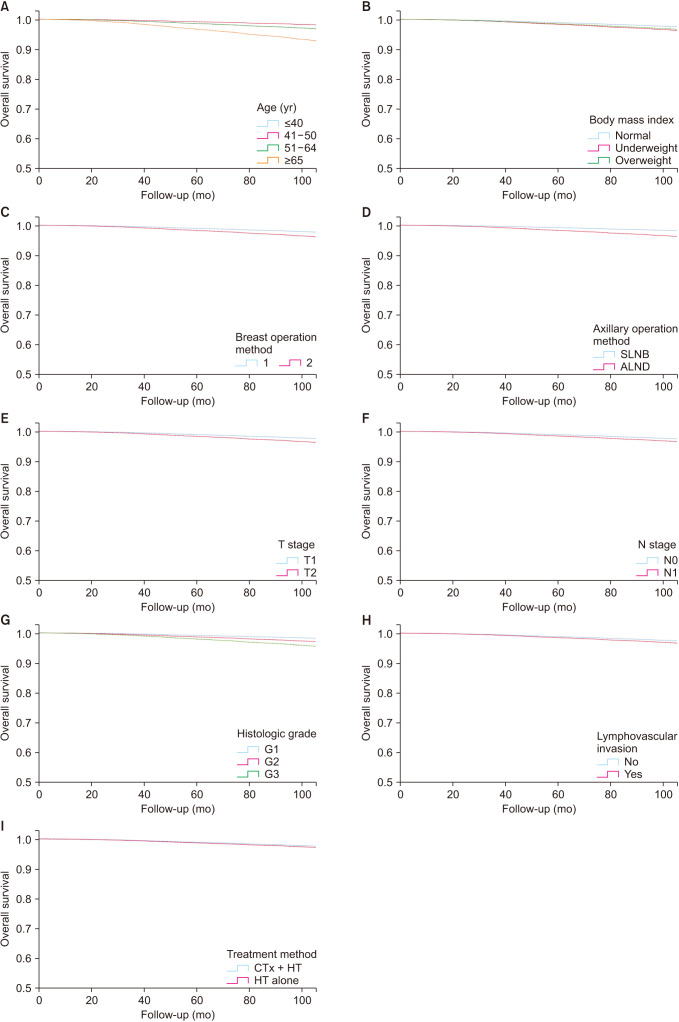
A total of 40,938 women were included in the study; 20,880 (51.0%) received CTx + HT, while 20,058 (49.0%) received HT only. In recent years, there has been a steady increase in the use of HT alone, from 21.0% (2000) to 64.6% (2018). In Cox regression analysis, age, type of breast and axillary operations, T and N stages, body mass index, histologic grade,and presence of lymphovascular invasion were prognostic indicators for OS. There was no significant difference between CTx + HT and HT alone in terms of OS (P = 0.126).
Conclusion
Over the years, there has been a shift from CTx + HT to HT alone without a significant difference in OS. Therefore, HT alone could be a safe treatment option in selected patients, even those with T2N1 disease.
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. PMID: 33538338.2. Jung KW, Won YJ, Kang MJ, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2022. Cancer Res Treat. 2022; 54:345–351. PMID: 35313101.3. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–344. PMID: 35313102.4. Thomssen C, Balic M, Harbeck N, Gnant M. St. Gallen/Vienna 2021: a brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care (Basel). 2021; 16:135–143. PMID: 34002112.5. National Comprehensive Cancer Network (NCCN). NCCN Guidelines, Breast Cancer (version 4) [Internet]. Plymouth Meeting (PA): NCCN;2022. cited 2022 Oct 7. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .6. Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014; 25:339–345. PMID: 24347518.7. Tang G, Shak S, Paik S, Anderson SJ, Costantino JP, Geyer CE Jr, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011; 127:133–142. PMID: 21221771.8. Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022; 40:1816–1837. PMID: 35439025.9. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, Zarella E, Binns S, Zhang Y, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res. 2016; 18:1. PMID: 26728744.10. Kang SY, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, et al. Breast cancer statistics in Korea in 2017: data from a Breast Cancer Registry. J Breast Cancer. 2020; 23:115–128. PMID: 32395372.11. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752. PMID: 10963602.12. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021; 385:2336–2347. PMID: 34914339.13. Haque W, Verma V, Hatch S, Klimberg VS, Butler EB, Teh BS. Omission of chemotherapy for low-grade, luminal A N1 breast cancer: patterns of care and clinical outcomes. Breast. 2018; 41:67–73. PMID: 30007270.14. Kim HS, Lee JU, Yoo TK, Chae BJ, Son D, Kim YJ, et al. Omission of chemotherapy for the treatment of mucinous breast cancer: a nationwide study from the Korean Breast Cancer Society. J Breast Cancer. 2019; 22:599–612. PMID: 31897333.15. Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000; 92:550–556. PMID: 10749910.16. Freyer G, Braud AC, Chaibi P, Spielmann M, Martin JP, Vilela G, et al. Dealing with metastatic breast cancer in elderly women: results from a French study on a large cohort carried out by the ‘Observatory on Elderly Patients’. Ann Oncol. 2006; 17:211–216. PMID: 16291586.17. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008; 26:2373–2378. PMID: 18413639.18. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013; 119:1402–1411. PMID: 23359049.19. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005; 16:1569–1583. PMID: 16148022.20. Gujam FJ, Going JJ, Edwards J, Mohammed ZM, McMillan DC. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol. 2014; 89:231–241. PMID: 24075309.21. Leitner SP, Swern AS, Weinberger D, Duncan LJ, Hutter RV. Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a,b N0 M0). Cancer. 1995; 76:2266–2274. PMID: 8635031.22. Mohammed RA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, et al. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol. 2011; 223:358–365. PMID: 21171081.23. Pinder SE, Ellis IO, Galea M, O’Rouke S, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology. 1994; 24:41–47. PMID: 8144141.24. Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam CM, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004; 15:875–884. PMID: 15151943.25. Bao PP, Cai H, Peng P, Gu K, Su Y, Shu XO, et al. Body mass index and weight change in relation to triple-negative breast cancer survival. Cancer Causes Control. 2016; 27:229–236. PMID: 26621544.26. Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008; 19:1319–1328. PMID: 18752034.27. Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012; 23:83–89. PMID: 22169755.28. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003; 95:1218–1226. PMID: 12928347.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hormone Treatment for Breast Cancer
- Oncologic Effect of Oral Fluorouracil in Hormone Receptor-Negative T1a Node-Negative Breast Cancer Patients
- Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
- The Prognostic Value of Epidermal Growth Factor Receptor in Primary Breast Cancer
- Adjuvant Trastuzumab Is Required in Human Epidermal Growth Factor Receptor 2-Positive Node-Negative Breast Cancer Patients Regardless of Tumour Size