Clin Exp Otorhinolaryngol.
2022 Nov;15(4):335-345. 10.21053/ceo.2021.01928.
Compositional Alterations of the Nasal Microbiome and Staphylococcus aureus–Characterized Dysbiosis in the Nasal Mucosa of Patients With Allergic Rhinitis
- Affiliations
-
- 1Department of Otorhinolaryngology, Seoul National University College of Medicine, Seoul, Korea
- 2Sensory Organ Research Institute, Seoul National University Medical Research Center, Seoul, Korea
- 3Department of Microbiology, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2536532
- DOI: http://doi.org/10.21053/ceo.2021.01928
Abstract
Objectives
. Host–microbial commensalism can shape the innate immune response in the nasal mucosa, and the microbial characteristics of nasal mucus directly impact the mechanisms of the initial allergic responses in the nasal epithelium. We sought to determine alterations of the microbial composition in the nasal mucus of patients with allergic rhinitis (AR) and to elucidate the interplay between dysbiosis of the nasal microbiome and allergic inflammation.
Methods
. In total, 364,923 high-quality bacterial 16S ribosomal RNA-encoding gene sequence reads from 104 middle turbinate mucosa samples from healthy participants and patients with AR were obtained and analyzed using the Quantitative Insights into Microbial Ecology pipeline.
Results
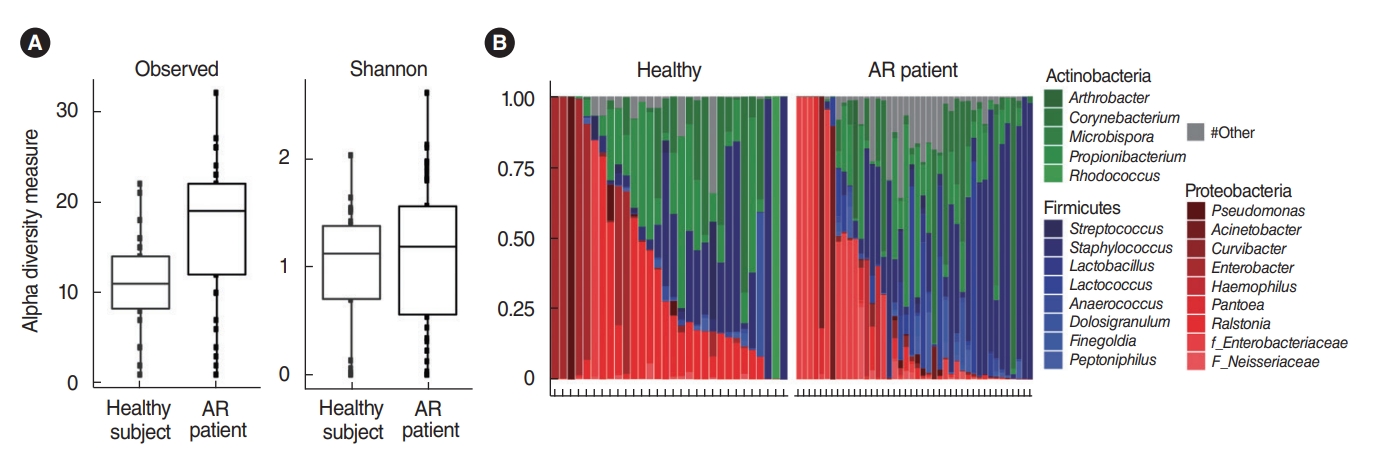
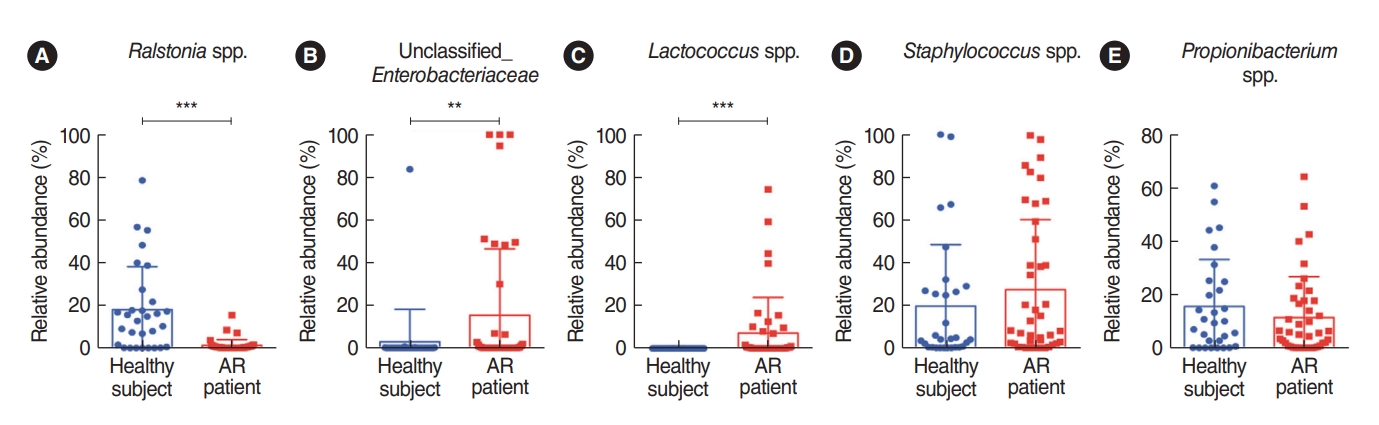
. We analyzed the microbiota in samples of nasal mucus from patients with AR (n=42) and clinically healthy participants (n=30). The Proteobacteria (Ralstonia genus) and Actinobacteria (Propionibacterium genus) phyla were predominant in the nasal mucus of healthy subjects, whereas the Firmicutes (Staphylococcus genus) phylum was significantly abundant in the nasal mucus of patients with AR. In particular, the Ralstonia genus was significantly dominant in the clinically healthy subjects. Additional pyrosequencing data from 32 subjects (healthy participants: n=15, AR patients: n=17) revealed a greater abundance of Staphylococcus epidermidis, Corynebacterium accolens, and Nocardia coeliaca, accounting for 41.55% of mapped sequences in the nasal mucus of healthy participants. Dysbiosis of the nasal microbiome was more pronounced in patients with AR, and Staphylococcus aureus exhibited the greatest abundance (37.69%) in their nasal mucus, in association with a positive response to house dust mites and patients’ age and height.
Conclusion
. This study revealed alterations in the nasal microbiome in the nasal mucus of patients with AR at the levels of microbial genera and species. S. aureus-dominant dysbiosis was distinctive in the nasal mucus of patients with AR, suggesting a role of host-microbial commensalism in allergic inflammation.
Keyword
Figure
Reference
-
1. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; Jul. 454(7203):445–54.
Article2. Khalmuratova R, Shin HW. Influence of the genetic background on allergic rhinitis models in mice. Clin Exp Otorhinolaryngol. 2020; Nov. 13(4):322–3.
Article3. Won J, Gil CH, Jo A, Kim HJ. Inhaled delivery of interferon-lambda restricts epithelial-derived Th2 inflammation in allergic asthma. Cytokine. 2019; Jul. 119:32–6.
Article4. Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015; Jul. 43(1):29–40.
Article5. Jeon YJ, Lim JH, An S, Jo A, Han DH, Won TB, et al. Type III interferons are critical host factors that determine susceptibility to influenza A viral infection in allergic nasal mucosa. Clin Exp Allergy. 2018; Mar. 48(3):253–65.
Article6. Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013; Jun. 14(7):676–84.
Article7. Kim HJ, Kim CH, Ryu JH, Kim MJ, Park CY, Lee JM, et al. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. Am J Respir Cell Mol Biol. 2013; Nov. 49(5):855–65.
Article8. Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS One. 2012; Jun. 7(6):e34242.
Article9. Lukacs NW, Huang YJ. Microbiota-immune interactions in asthma pathogenesis and phenotype. Curr Opin Immunol. 2020; Oct. 66:22–6.
Article10. Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol. 2019; Sep. 20(10):1279–90.
Article11. Bender ME, Read TD, Edwards TS, Hargita M, Cutler AJ, Wissel EF, et al. A comparison of the bacterial nasal microbiome in allergic rhinitis patients before and after immunotherapy. Laryngoscope. 2020; Dec. 130(12):E882–8.
Article12. Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol. 2014; Nov. 14(12):827–35.
Article13. Kim DK, Lee BC, Park KJ, Son GM. Effect of obstructive sleep apnea on immunity in cases of chronic rhinosinusitis with nasal polyps. Clin Exp Otorhinolaryngol. 2021; Nov. 14(4):390–8.
Article14. Cho SW, Yang SK. What does the microbiome in the tonsil tell us. Clin Exp Otorhinolaryngol. 2021; Aug. 14(3):247–8.
Article15. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010; Jan. 5(1):e8578.
Article16. Martin C, Burgel PR, Lepage P, Andrejak C, de Blic J, Bourdin A, et al. Host-microbe interactions in distal airways: relevance to chronic airway diseases. Eur Respir Rev. 2015; Mar. 24(135):78–91.
Article17. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; May. 7(5):335–6.
Article18. McMurdie PJ, Holmes S. Shiny-phyloseq: web application for interactive microbiome analysis with provenance tracking. Bioinformatics. 2015; Jan. 31(2):282–3.
Article19. Zhang H, Lu N, Feng C, Thurston SW, Xia Y, Zhu L, et al. On fitting generalized linear mixed-effects models for binary responses using different statistical packages. Stat Med. 2011; Sep. 30(20):2562–72.
Article20. Kim HJ, Jo A, Jeon YJ, An S, Lee KM, Yoon SS, et al. Nasal commensal staphylococcus epidermidis enhances interferon-λ-dependent immunity against influenza virus. Microbiome. 2019; May. 7(1):80.
Article21. Park HK, Ha MH, Park SG, Kim MN, Kim BJ, Kim W. Characterization of the fungal microbiota (mycobiome) in healthy and dandruffafflicted human scalps. PLoS One. 2012; Feb. 7(2):e32847.
Article22. Ramakrishnan VR, Hauser LJ, Frank DN. The sinonasal bacterial microbiome in health and disease. Curr Opin Otolaryngol Head Neck Surg. 2016; Feb. 24(1):20–5.
Article23. Ramakrishnan VR, Hauser LJ, Feazel LM, Ir D, Robertson CE, Frank DN. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J Allergy Clin Immunol. 2015; Aug. 136(2):334–42.e1.
Article24. Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, et al. Sinus microbiome diversity depletion and corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012; Sep. 4(151):151ra124.25. Jeon YJ, Gil CH, Won J, Jo A, Kim HJ. Symbiotic microbiome Staphylococcus aureus from human nasal mucus modulates IL-33-mediated type 2 immune responses in allergic nasal mucosa. BMC Microbiol. 2020; Oct. 20(1):301.
Article26. Khalmuratova R, Shin HW. Crosstalk between mucosal inflammation and bone metabolism in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2021; Feb. 14(1):43–9.
Article27. Park SC, Park IH, Lee JS, Park SM, Kang SH, Hong SM, et al. Microbiome of unilateral chronic rhinosinusitis: a controlled paired analysis. Int J Environ Res Public Health. 2021; Sep. 18(18):9878.
Article28. Riechelmann H, Essig A, Deutschle T, Rau A, Rothermel B, Weschta M. Nasal carriage of Staphylococcus aureus in house dust mite allergic patients and healthy controls. Allergy. 2005; Nov. 60(11):1418–23.
Article29. Kim DH, Kim SW. Considerations for the use of biologic agents in patients with chronic rhinosinusitis with nasal polyposis. Clin Exp Otorhinolaryngol. 2021; Aug. 14(3):245–6.
Article30. Kim YC, Won HK, Lee JW, Sohn KH, Kim MH, Kim TB, et al. Staphylococcus aureus nasal colonization and asthma in adults: systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2019; Feb. 7(2):606–15.e9.
Article31. Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015; Apr. 42(4):756–66.
Article32. Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature. 2013; Nov. 503(7476):397–401.
Article33. Hyun DW, Min HJ, Kim MS, Whon TW, Shin NR, Kim PS, et al. Dysbiosis of inferior turbinate microbiota is associated with high total IgE levels in patients with allergic rhinitis. Infect Immun. 2018; Mar. 86(4):e00934–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Correlation Study between the Specific IgE for Staphylococcus Aureus Exotoxin and Nasal Mucus Culture in Allergic Rhinitis
- Upregulation of the Vitamin D Receptor in the Nasal Mucosa of Patients With Allergic Rhinitis
- Evidences for Local Allergic Rhinitis
- Diagnosis of Allergic Rhinitis
- Detection of Staphylococcus Aureus Exotoxins(SEA, TSST-1) in Nasal Polyp with Chronic Rhinosinusitis Patients