Nutr Res Pract.
2022 Dec;16(6):700-715. 10.4162/nrp.2022.16.6.700.
Differential effects of various dietary proteins on dextran sulfate sodiuminduced colitis in mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Daegu Catholic University, Gyeongsan 38430, Korea
- 2Gyeongsangbuk-do Institute of Health & Environment, Yeongcheon 38874, Korea
- KMID: 2536505
- DOI: http://doi.org/10.4162/nrp.2022.16.6.700
Abstract
- BACKGROUND/OBJECTIVES
Chronic colitis is a risk factor for colorectal cancer (CRC) development in both animals and humans. Previously, we reported that a diet rich in protein (with casein as the protein source) significantly increased the risk of mouse CRC development in a dose-dependent manner. In this study, we investigated the effects of different protein sources on the risk of colitis development.
MATERIALS/METHODS
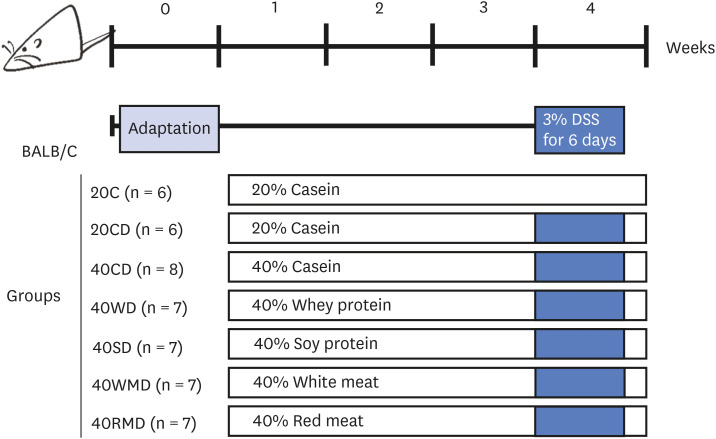
Balb/c mice were divided into 7 experimental groups: 20% casein (20C), 20C-dextran sulfate sodium (DSS), 40% casein-DSS (40CD), 40% whey protein-DSS (40WD), 40% soy protein-DSS (40SD), 40% white meat-DSS (40WMD), and 40% red meatDSS (40RMD). Mice were fed an experimental diet for 4 wk and received 3% DSS in their drinking water for 6 days during the 4th wk of the experimental period.
RESULTS
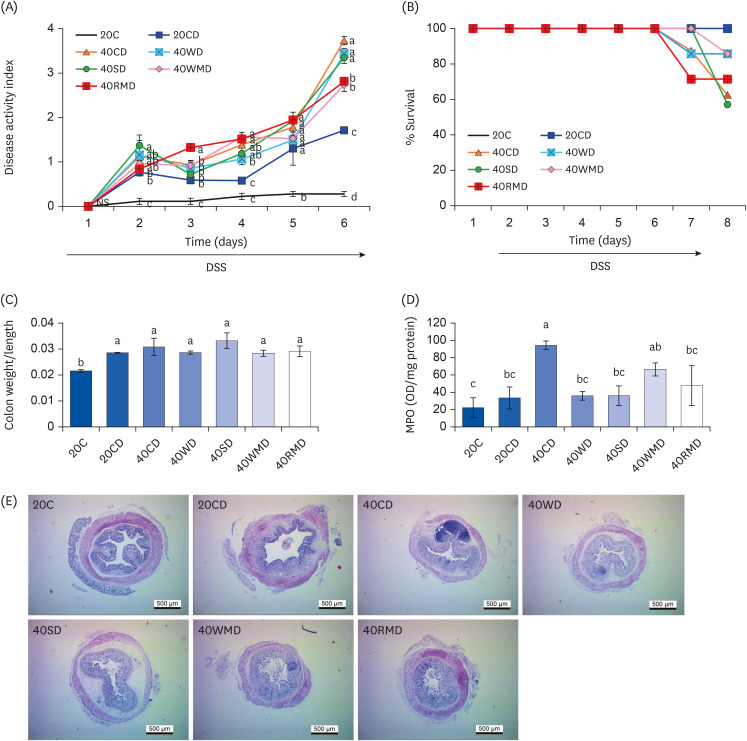
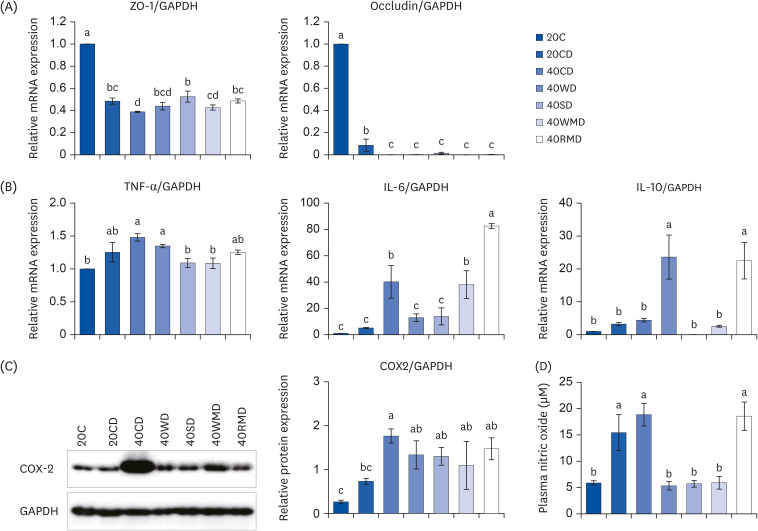
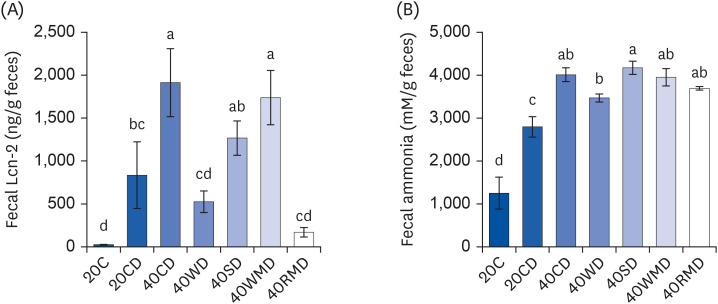
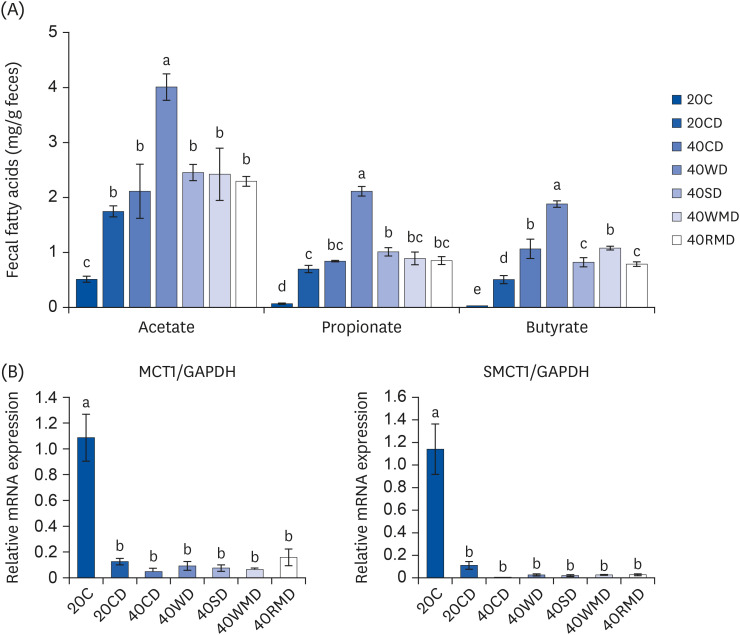
Compared to other groups, the 40CD group showed the most aggravated colitis with increased disease activity and inflammatory markers. In the 40RMD group, interleukin (IL)-6 levels were the highest among all the groups. The 40SD group showed conflicting effects, for example, elevated mortality and disease activity but decreased nitric oxide (NO) levels. The 40WD group showed attenuated colitis with increased IL-10 levels and decreased NO levels. The 40WMD group showed conflicting effects, including decreased NO levels and elevated fecal lipocalin-2 and IL-6 levels.
CONCLUSIONS
These results suggest that, at levels of 40% in the diet, casein and red meat exacerbate colitis, whereas whey protein mitigates it the most effectively.
Keyword
Figure
Reference
-
1. Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2010; 6:339–346. PMID: 20567592.2. Keshteli AH, Madsen KL, Dieleman LA. Diet in the pathogenesis and management of ulcerative colitis; a review of randomized controlled dietary interventions. Nutrients. 2019; 11:1498. PMID: 31262022.
Article3. Benchimol EI, Mack DR, Guttmann A, Nguyen GC, To T, Mojaverian N, Quach P, Manuel DG. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015; 110:553–563. PMID: 25756238.
Article4. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444. PMID: 18650914.
Article5. Kim YH, Kwon HS, Kim DH, Cho HJ, Lee HS, Jun JG, Park JHY, Kim JK. Piceatannol, a stilbene present in grapes, attenuates dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2008; 8:1695–1702. PMID: 18773974.
Article6. Byun SY, Kim DB, Kim E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr Res. 2015; 35:726–735. PMID: 26094212.
Article7. Lan A, Blais A, Coelho D, Capron J, Maarouf M, Benamouzig R, Lancha AH Jr, Walker F, Tomé D, Blachier F. Dual effects of a high-protein diet on DSS-treated mice during colitis resolution phase. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G624–G633. PMID: 27562061.
Article8. Tak KH, Ahn E, Kim E. Increase in dietary protein content exacerbates colonic inflammation and tumorigenesis in azoxymethane-induced mouse colon carcinogenesis. Nutr Res Pract. 2017; 11:281–289. PMID: 28765774.
Article9. Vidal-Lletjós S, Beaumont M, Tomé D, Benamouzig R, Blachier F, Lan A. Dietary protein and amino acid supplementation in inflammatory bowel disease course: what impact on the colonic mucosa? Nutrients. 2017; 9:310. PMID: 28335546.
Article10. van Nuenen MH, Venema K, van der Woude JC, Kuipers EJ. The metabolic activity of fecal microbiota from healthy individuals and patients with inflammatory bowel disease. Dig Dis Sci. 2004; 49:485–491. PMID: 15139503.
Article11. Jiang H, Przybyszewski J, Mitra D, Becker C, Brehm-Stecher B, Tentinger A, MacDonald RS. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-α in colon of dextran sodium sulfate-treated C57BL/6 mice. J Nutr. 2011; 141:1239–1246. PMID: 21593350.
Article12. McIntosh GH, Regester GO, Le Leu RK, Royle PJ, Smithers GW. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J Nutr. 1995; 125:809–816. PMID: 7722681.13. Belobrajdic DP, McIntosh GH, Owens JA. Whey proteins protect more than red meat against azoxymethane induced ACF in Wistar rats. Cancer Lett. 2003; 198:43–51. PMID: 12893429.
Article14. Pandurangan AK, Mohebali N, Norhaizan ME, Looi CY. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des Devel Ther. 2015; 9:3923–3934.
Article15. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45. PMID: 11328886.
Article16. Han X, Guo J, You Y, Yin M, Ren C, Zhan J, Huang W. A fast and accurate way to determine short chain fatty acids in mouse feces based on GC-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2018; 1099:73–82.
Article17. Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012; 7:e44328. PMID: 22957064.
Article18. Mouillé B, Robert V, Blachier F. Adaptative increase of ornithine production and decrease of ammonia metabolism in rat colonocytes after hyperproteic diet ingestion. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G344–G351. PMID: 15064231.
Article19. Topping DC, Visek WJ. Synthesis of macromolecules by intestinal cells incubated with ammonia. Am J Physiol. 1977; 233:E341–E347. PMID: 910948.
Article20. Ichikawa H, Sakata T. Stimulation of epithelial cell proliferation of isolated distal colon of rats by continuous colonic infusion of ammonia or short-chain fatty acids is nonadditive. J Nutr. 1998; 128:843–847. PMID: 9566991.
Article21. Cremin JD Jr, Fitch MD, Fleming SE. Glucose alleviates ammonia-induced inhibition of short-chain fatty acid metabolism in rat colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003; 285:G105–G114. PMID: 12637251.
Article22. Le Leu RK, Young GP, Hu Y, Winter J, Conlon MA. Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation. Dig Dis Sci. 2013; 58:3475–3482. PMID: 23990000.
Article23. McIntosh GH, Le Leu RK. The influence of dietary proteins on colon cancer risk. Nutr Res. 2001; 21:1053–1066. PMID: 11446989.
Article24. Shi J, Zhao D, Song S, Zhang M, Zamaratskaia G, Xu X, Zhou G, Li C. High-meat-protein high-fat diet induced dysbiosis of gut microbiota and tryptophan metabolism in Wistar rats. J Agric Food Chem. 2020; 68:6333–6346. PMID: 32432868.
Article25. Ronis MJ, Hakkak R, Korourian S, Badger TM. Whey protein hydrolysate but not whole whey protein protects against 7, 12-dimethylbenz (a) anthracene-induced mammary tumors in rats. Nutr Cancer. 2015; 67:949–953. PMID: 26168336.
Article26. Yu Y, Jing X, Li H, Zhao X, Wang D. Soy isoflavone consumption and colorectal cancer risk: a systematic review and meta-analysis. Sci Rep. 2016; 6:25939. PMID: 27170217.
Article27. Guo YW, Chen YH, Chiu WC, Liao H, Lin SH. Soy saponins meditate the progression of colon cancer in rats by inhibiting the activity of β-glucuronidase and the number of aberrant crypt foci but not cyclooxygenase-2 activity. ISRN Oncol. 2013; 2013:645817. PMID: 24224098.28. Govers MJ, Lapré JA, De Vries HT, Van der Meer R. Dietary soybean protein compared with casein damages colonic epithelium and stimulates colonic epithelial proliferation in rats. J Nutr. 1993; 123:1709–1713. PMID: 8410362.
Article29. Pan L, Farouk MH, Qin G, Zhao Y, Bao N. The influences of soybean agglutinin and functional oligosaccharides on the intestinal tract of monogastric animals. Int J Mol Sci. 2018; 19:554. PMID: 29439523.
Article30. Badger TM, Ronis MJ, Hakkak R. Developmental effects and health aspects of soy protein isolate, casein, and whey in male and female rats. Int J Toxicol. 2001; 20:165–174. PMID: 11488559.
Article31. Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila). 2011; 4:1903–1911. PMID: 21803982.
Article32. Ollberding NJ, Wilkens LR, Henderson BE, Kolonel LN, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: the Multiethnic Cohort Study. Int J Cancer. 2012; 131:E1125–E1133. PMID: 22438055.
Article33. Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2015; 112:542–547. PMID: 25548184.
Article34. Zhu Y, Lin X, Zhao F, Shi X, Li H, Li Y, Zhu W, Xu X, Li C, Zhou G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci Rep. 2015; 5:15220. PMID: 26463271.
Article35. Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007; 133:1916–1927. PMID: 18054563.
Article36. Kostovcikova K, Coufal S, Galanova N, Fajstova A, Hudcovic T, Kostovcik M, Prochazkova P, Jiraskova Zakostelska Z, Cermakova M, Sediva B, et al. Diet rich in animal protein promotes pro-inflammatory macrophage response and exacerbates colitis in mice. Front Immunol. 2019; 10:919. PMID: 31105710.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of mulberry twig extracts on dextran sulfate sodium-induced colitis mouse model
- Long-Term Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Dextran Sulfate Sodium-Induced Murine Chronic Colitis
- Fine Structure of Goblet Cell Regeneration on Experimental Colitis Induced by Dextran Sulfate Sodium
- Histological Study of Experimental Colitis Induced by Dextran Sulfate Sodium
- Th17 Responses Are Not Induced in Dextran Sodium Sulfate Model of Acute Colitis