Int J Stem Cells.
2022 Nov;15(4):359-371. 10.15283/ijsc21135.
MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- Affiliations
-
- 1Department of Plastic Surgery and Medical Cosmetology, Hainan Cancer Hospital, Haikou, Hainan, China
- KMID: 2536206
- DOI: http://doi.org/10.15283/ijsc21135
Abstract
- Background and Objectives
The goal of this study was to investigate the mechanism of mesenchymal stem cell (MSC)-derived microRNA (miR)-150-5p-expressing exosomes in promoting skin wound healing through activating PI3K/AKT pathway by PTEN.
Methods and Results
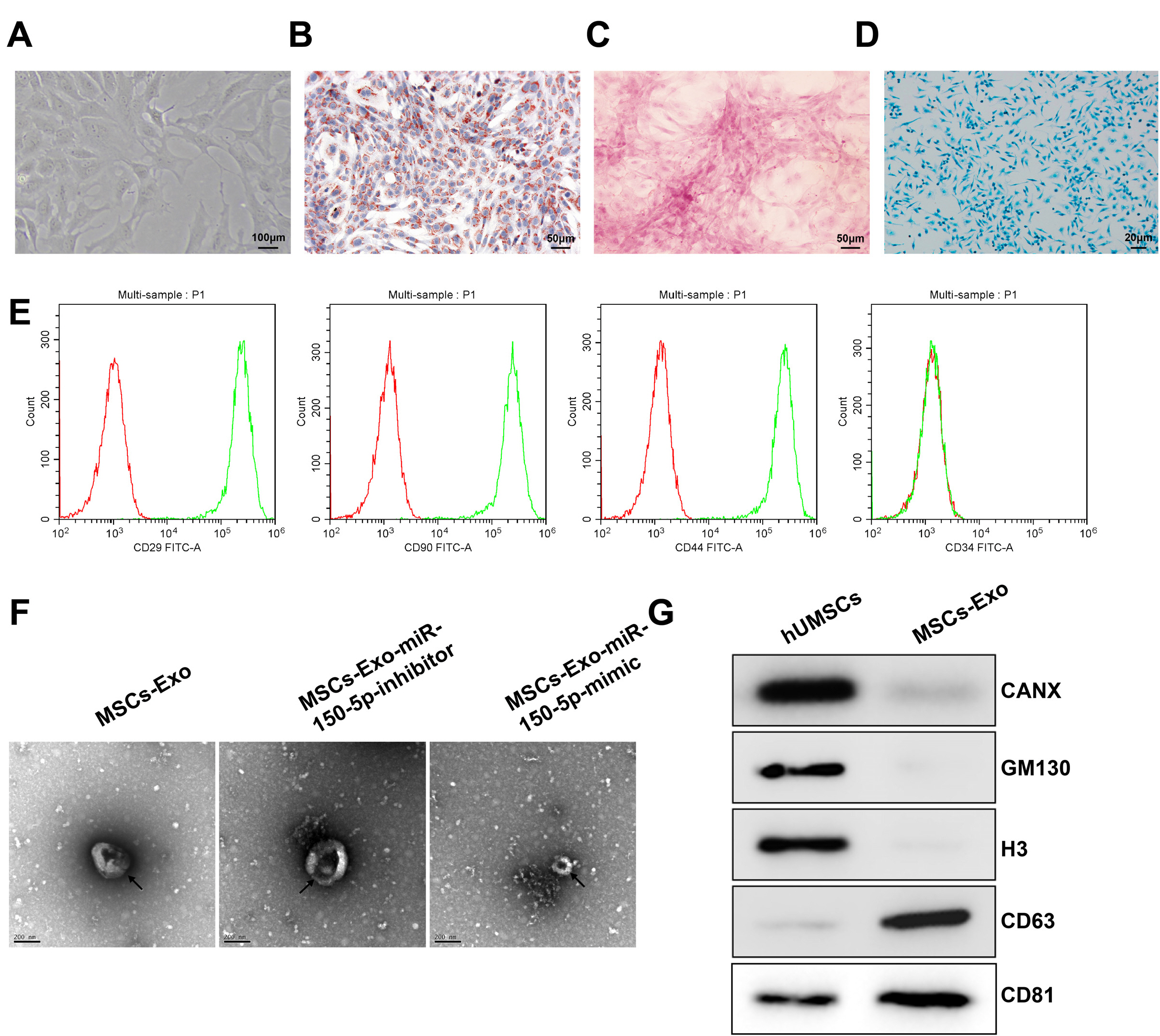
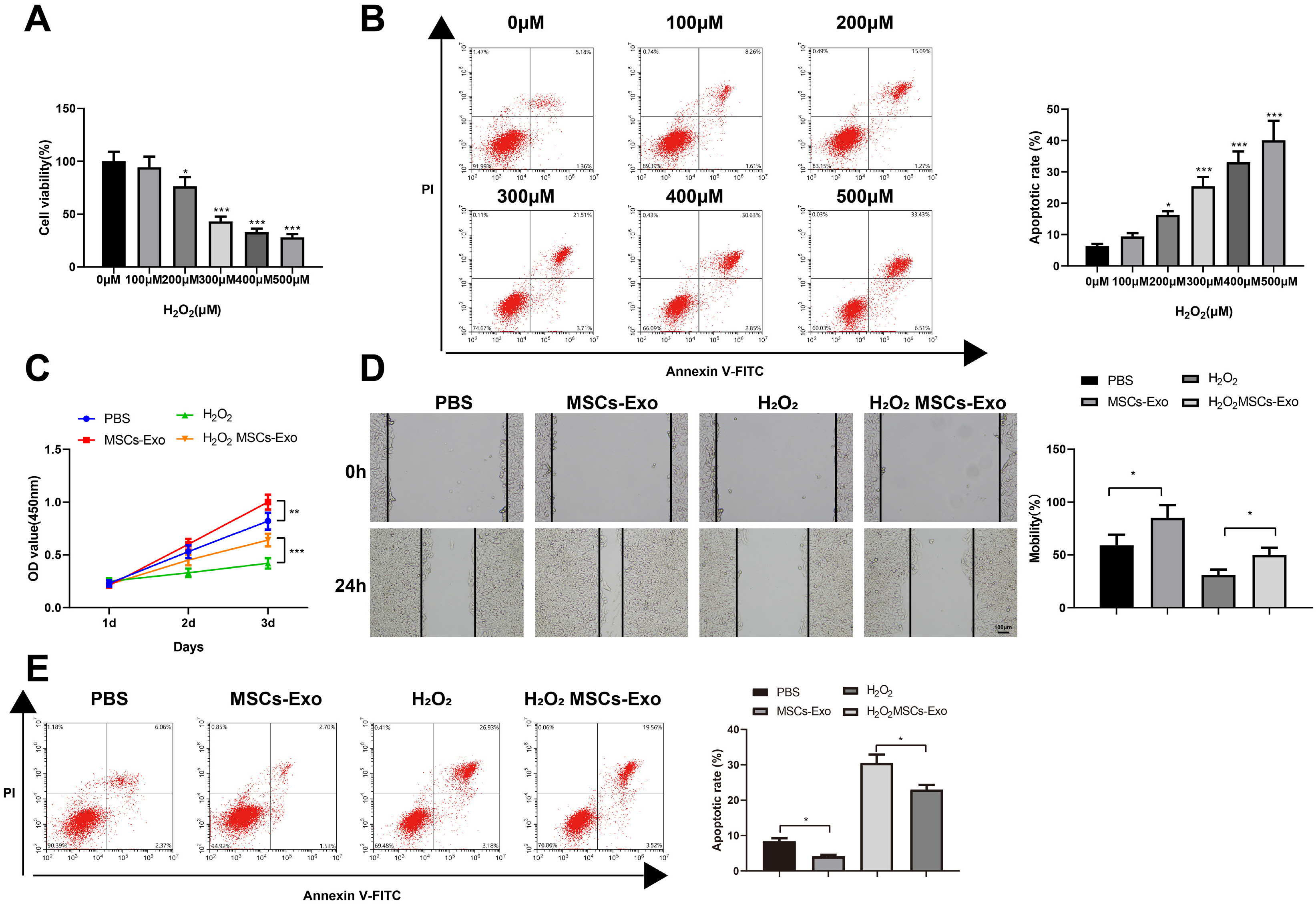
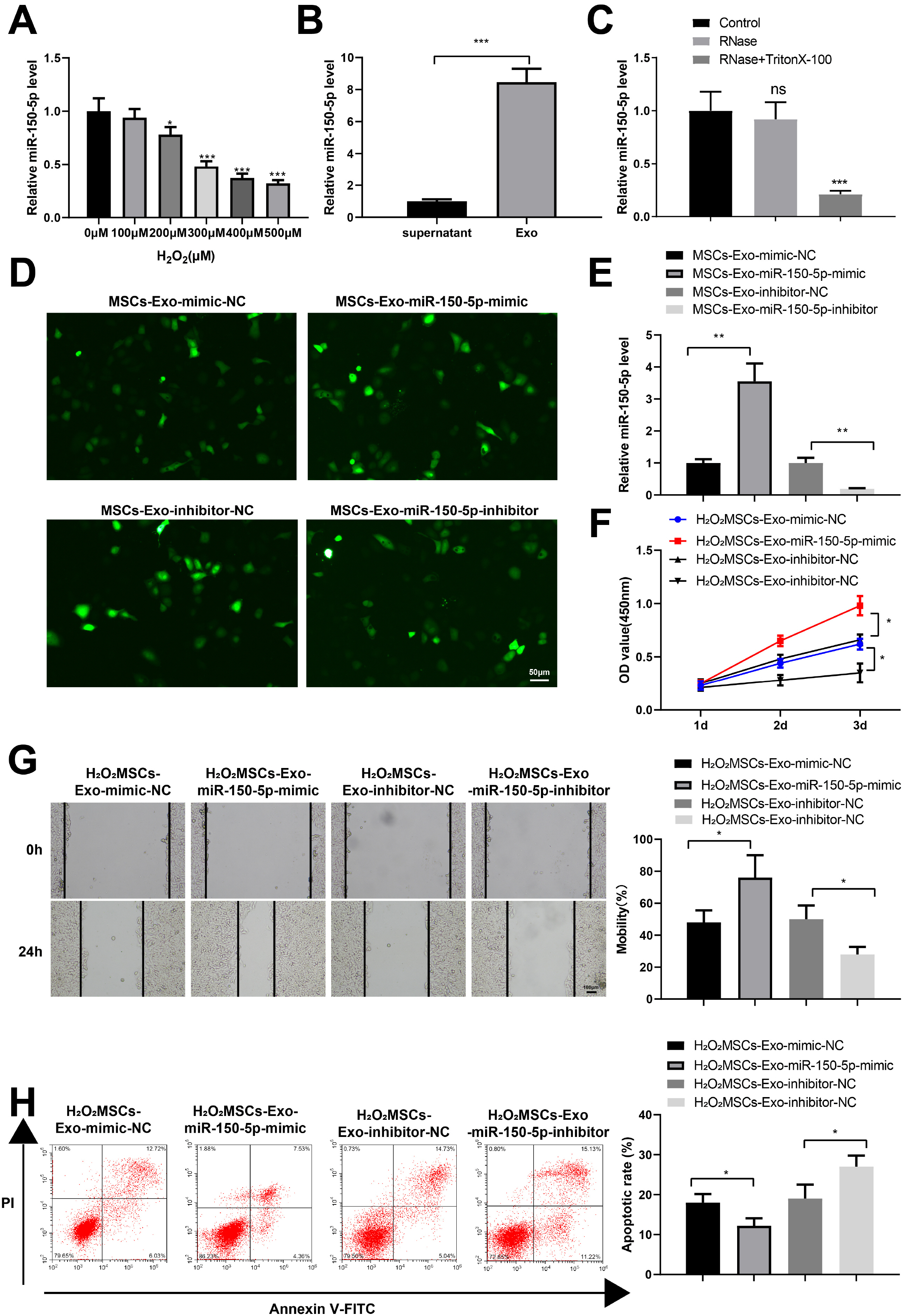
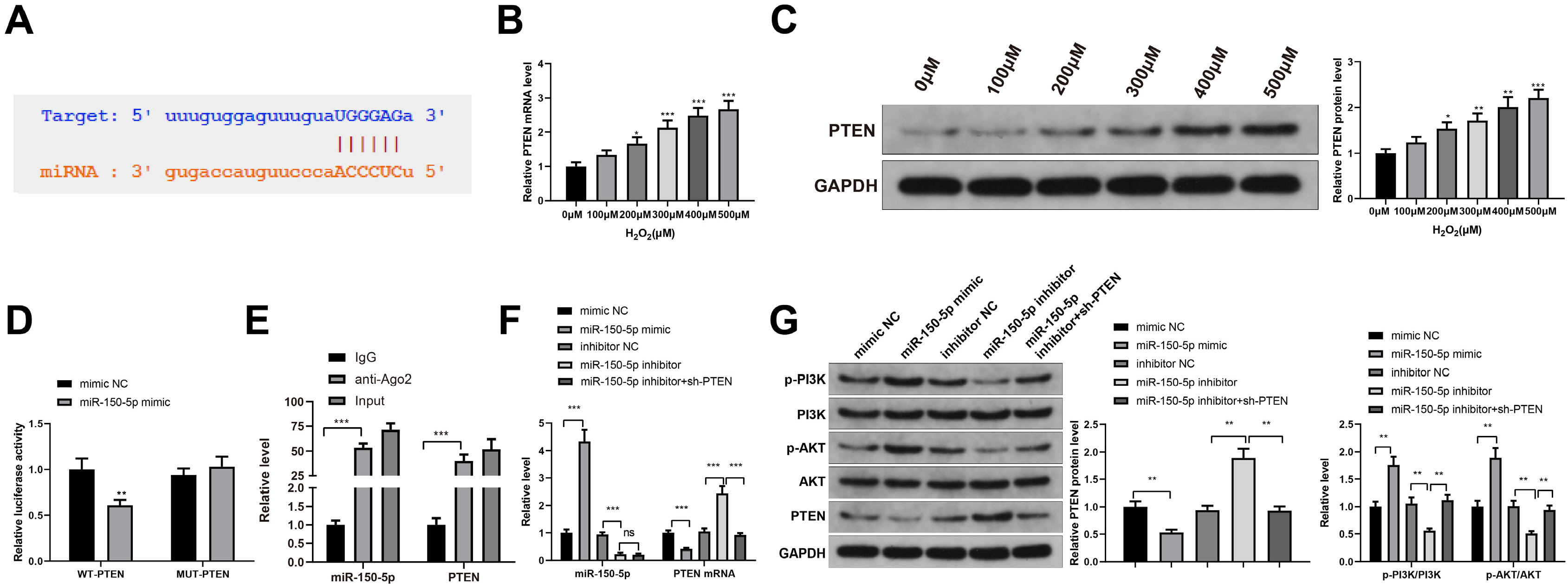
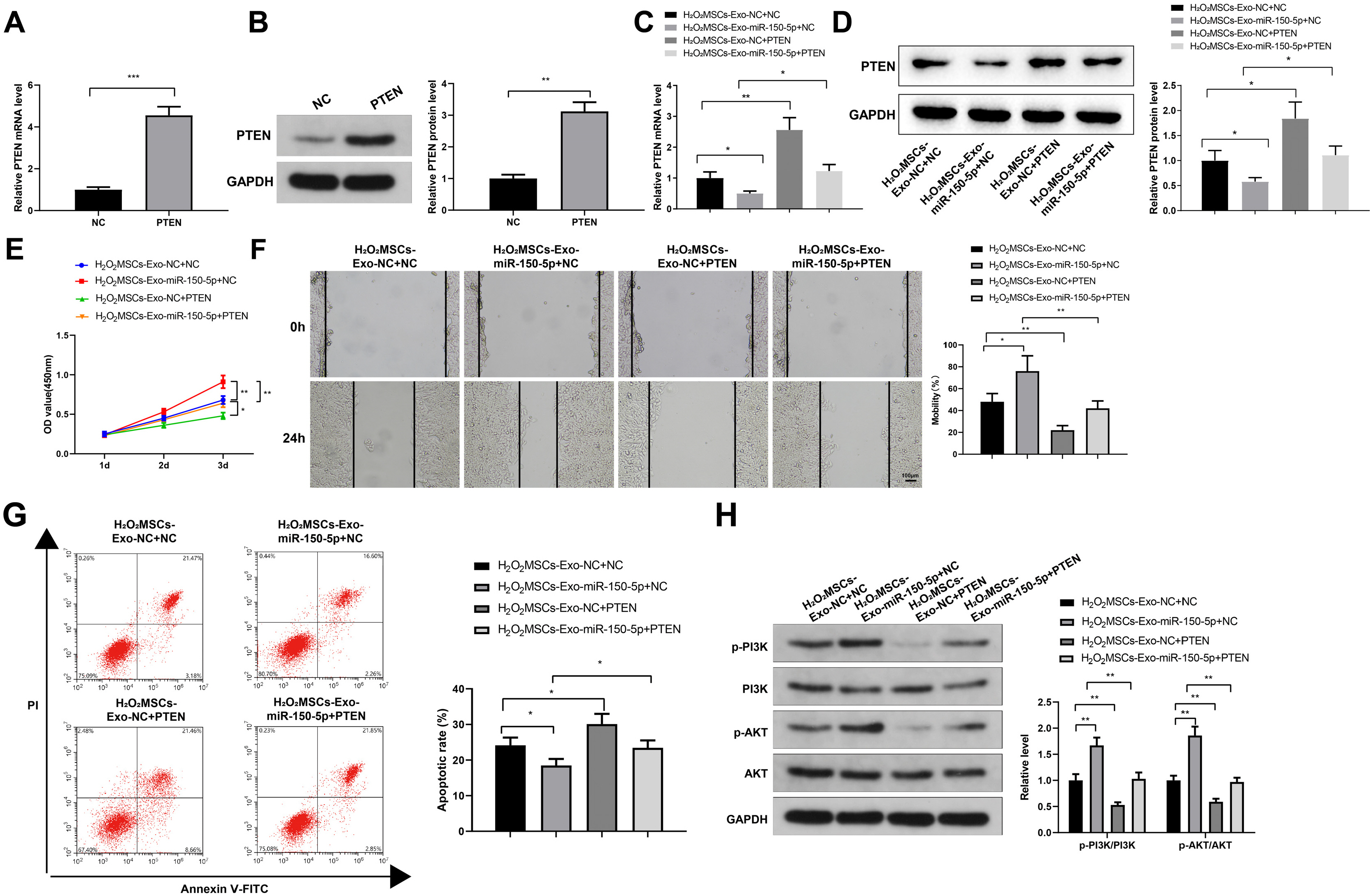
Human umbilical cord (HUC)-MSCs were infected with miR-150-5p overexpression and its con-trol lentivirus, and HUC-MSCs-derived exosomes (MSCs-Exos) with stable expression of miR-150-5p were obtained. HaCaT cells were induced by H2O2 to establish a cellular model of skin injury, in which the expression of miR-150-5p and PTEN and the phosphorylation of PI3K and AKT were evaluated. HaCaT cells were transfected with pcDNA3.1-PTEN or pcDNA3.1 and then cultured with normal exosomes or exosomes stably expressing miR-150-5p. Cell proliferation was inspected by CCK-8. Cell migration was detected by scratch test and cell apoptosis by flow cytometry. The starBase tool was used to predict the binding site of miR-150-5p to PTEN. Dual-luciferase reporter assay and RIP assay were applied to assess the interaction between miR-150-5p and PTEN. In H2O2 -induced HaCaT cells, the miR-150-5p expression decreased, and PTEN expression increased in a concentration-dependent manner. MSCs-Exos promoted the growth and migration of H2O2 -induced HaCaT cells and inhibited their apoptosis. In addition, overexpression of exosomal miR-150-5p enhanced the protective effect of MSCs-Exos on H2O2 -induced HaCaT cells; PTEN overexpression in HaCaT cells partially restrained miR-150-5p-mediated inhibition on H2O2 -induced injury in HaCaT cells. PTEN was a target gene of miR-150-5p. MiR-150-5p regulated PI3K/AKT pathway through PTEN.
Conclusions
MSCs-derived miR-150-5p-expressing exosomes promote skin wound healing by activating PI3K/AKT pathway through PTEN.
Keyword
Figure
Reference
-
References
1. Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. 2015; Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 13:49. DOI: 10.1186/s12967-015-0417-0. PMID: 25638205. PMCID: PMC4371881. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84925338126&origin=inward.
Article2. Cui HS, Joo SY, Cho YS, Park JH, Kim JB, Seo CH. 2020; Effect of combining low temperature plasma, negative pressure wound therapy, and bone marrow mesenchymal stem cells on an acute skin wound healing mouse model. Int J Mol Sci. 21:3675. DOI: 10.3390/ijms21103675. PMID: 32456187. PMCID: PMC7279345. PMID: fcb2542e5ce54a94b00d18049b08a695. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085469497&origin=inward.
Article3. Falanga V. 2005; Wound healing and its impairment in the diabetic foot. Lancet. 366:1736–1743. DOI: 10.1016/S0140-6736(05)67700-8. PMID: 16291068. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=27744448125&origin=inward.
Article4. Ennis WJ, Sui A, Bartholomew A. 2013; Stem cells and healing: impact on inflammation. Adv Wound Care (New Rochelle). 2:369–378. DOI: 10.1089/wound.2013.0449. PMID: 24587974. PMCID: PMC3842880.
Article5. Oh SY, Lee SJ, Jung YH, Lee HJ, Han HJ. 2015; Arachidonic acid promotes skin wound healing through induction of human MSC migration by MT3-MMP-mediated fibronectin degradation. Cell Death Dis. 6:e1750. DOI: 10.1038/cddis.2015.114. PMID: 25950480. PMCID: PMC4669694. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84953371939&origin=inward.
Article6. Frykberg RG, Banks J. 2015; Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 4:560–582. DOI: 10.1089/wound.2015.0635. PMID: 26339534. PMCID: PMC4528992.
Article7. Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, Zhang C, Li Q, Wang Y. 2016; Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 12:1472–1487. DOI: 10.7150/ijbs.15514. PMID: 27994512. PMCID: PMC5166489. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84997552914&origin=inward.
Article8. Phinney DG, Pittenger MF. 2017; Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 35:851–858. Erratum in: Stem Cells 2017;35:2103. DOI: 10.1002/stem.2575. PMID: 28294454. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85016131316&origin=inward.
Article9. Ti D, Hao H, Fu X, Han W. 2016; Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflamma-tion. Sci China Life Sci. 59:1305–1312. DOI: 10.1007/s11427-016-0240-4. PMID: 27864711. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84995783647&origin=inward.
Article10. Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. 2015; HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 33:2158–2168. Erratum in: Stem Cells 2021;39:E5. DOI: 10.1002/stem.1771. PMID: 24964196. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84931578273&origin=inward.
Article11. Lässer C. 2012; Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin Biol Ther. 12 Suppl 1:S189–S197. DOI: 10.1517/14712598.2012.680018. PMID: 22506888. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84861419046&origin=inward.
Article12. Duan M, Zhang Y, Zhang H, Meng Y, Qian M, Zhang G. 2020; Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res Ther. 11:452. DOI: 10.1186/s13287-020-01971-6. PMID: 33097078. PMCID: PMC7584097. PMID: 54dcc8c7c9e443ebafd9f8115fe0d018. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85093929187&origin=inward.
Article13. Gao S, Chen T, Hao Y, Zhang F, Tang X, Wang D, Wei Z, Qi J. 2020; Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res Ther. 11:56. DOI: 10.1186/s13287-020-1570-9. PMID: 32054526. PMCID: PMC7020560. PMID: 86a5eecbd87d4b098152db98e8e0f837. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079335702&origin=inward.
Article14. Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, Li HM, Zhang WS, Chen CY, Xie H. 2018; Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 8:169–184. DOI: 10.7150/thno.21234. PMID: 29290800. PMCID: PMC5743467. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85035087044&origin=inward.
Article15. Henriques-Antunes H, Cardoso RMS, Zonari A, Correia J, Leal EC, Jiménez-Balsa A, Lino MM, Barradas A, Kostic I, Gomes C, Karp JM, Carvalho E, Ferreira L. 2019; The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano. 13:8694–8707. DOI: 10.1021/acsnano.9b00376. PMID: 31390518. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071672028&origin=inward.
Article16. Cao L, Graue-Hernandez EO, Tran V, Reid B, Pu J, Mannis MJ, Zhao M. 2011; Downregulation of PTEN at corneal wound sites accelerates wound healing through increased cell migration. Invest Ophthalmol Vis Sci. 52:2272–2278. DOI: 10.1167/iovs.10-5972. PMID: 21212174. PMCID: PMC3080732. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955980980&origin=inward.
Article17. Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M. 2018; Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci Rep. 8:15173. DOI: 10.1038/s41598-018-33169-5. PMID: 30310159. PMCID: PMC6182003. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054775520&origin=inward.
Article18. Cui W, Dai J, Ma J, Gu H. 2019; circCDYL/microRNA-105-5p participates in modulating growth and migration of colon cancer cells. Gen Physiol Biophys. 38:485–495. DOI: 10.4149/gpb_2019037. PMID: 31829306.
Article19. Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, Shang JL, Gao CF, Zhang FR, Wang F, Sun SH. 2011; Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 54:1679–1689. DOI: 10.1002/hep.24563. PMID: 21769904. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80055046798&origin=inward.
Article20. Cardoso RMS, Rodrigues SC, Gomes CF, Duarte FV, Romao M, Leal EC, Freire PC, Neves R, Simões-Correia J. 2021; Development of an optimized and scalable method for isolation of umbilical cord blood-derived small extracellular vesicles for future clinical use. Stem Cells Transl Med. 10:910–921. DOI: 10.1002/sctm.20-0376. PMID: 33577723. PMCID: PMC8133342. PMID: e1f2c32bd9914a6886fa4ef15b781b83. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100860521&origin=inward.
Article21. Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008; Wound repair and regeneration. Nature. 453:314–321. DOI: 10.1038/nature07039. PMID: 18480812. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=43749088730&origin=inward.
Article22. Caplan AI, Correa D. 2011; The MSC: an injury drugstore. Cell Stem Cell. 9:11–15. DOI: 10.1016/j.stem.2011.06.008. PMID: 21726829. PMCID: PMC3144500. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79959951133&origin=inward.
Article23. Wu P, Zhang B, Shi H, Qian H, Xu W. 2018; MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 20:291–301. DOI: 10.1016/j.jcyt.2017.11.002. PMID: 29434006. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041742699&origin=inward.
Article24. Born LJ, Chang KH, Shoureshi P, Lay F, Bengali S, Hsu ATW, Abadchi SN, Harmon JW, Jay SM. 2022; HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv Healthc Mater. 11:e2002070. DOI: 10.1002/adhm.202002070. PMID: 33870645. PMCID: PMC8522167. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104349160&origin=inward.
Article25. Huang J, Yu M, Yin W, Liang B, Li A, Li J, Li X, Zhao S, Liu F. 2021; Development of a novel RNAi therapy: engineered miR-31 exosomes promoted the healing of diabetic wounds. Bioact Mater. 6:2841–2853. DOI: 10.1016/j.bioactmat.2021.02.007. PMID: 33718666. PMCID: PMC7905076. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85101147519&origin=inward.
Article26. Wu Z, Li W, Li J, Zhang Y, Zhang X, Xu Y, Hu Y, Li Q, Sun Q, Ma Z. 2020; Higher expression of miR-150-5p promotes tumorigenesis by suppressing LKB1 in non-small cell lung cancer. Pathol Res Pract. 216:153145. DOI: 10.1016/j.prp.2020.153145. PMID: 32827803. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089551622&origin=inward.
Article27. Chen Z, Wang H, Xia Y, Yan F, Lu Y. 2018; Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 201:2472–2482. DOI: 10.4049/jimmunol.1800304. PMID: 30224512. PMCID: PMC6176104. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054780305&origin=inward.
Article28. Wu Z, Cheng S, Wang S, Li W, Liu J. 2021; BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int Immunopharmacol. 93:107389. DOI: 10.1016/j.intimp.2021.107389. PMID: 33582480. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100602810&origin=inward.29. Malek M, Kielkowska A, Chessa T, Anderson KE, Barneda D, Pir P, Nakanishi H, Eguchi S, Koizumi A, Sasaki J, Juvin V, Kiselev VY, Niewczas I, Gray A, Valayer A, Spensberger D, Imbert M, Felisbino S, Habuchi T, Beinke S, Cosulich S, Le Novère N, Sasaki T, Clark J, Hawkins PT, Stephens LR. 2017; PTEN regulates PI(3,4)P2 signaling downstream of class I PI3K. Mol Cell. 68:566–580.e10. DOI: 10.1016/j.molcel.2017.09.024. PMID: 29056325. PMCID: PMC5678281. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85031821662&origin=inward.
Article30. Zhang W, Yu F, Yan C, Shao C, Gu P, Fu Y, Sun H, Fan X. 2020; PTEN inhibition accelerates corneal endothelial wound healing through increased endothelial cell division and migration. Invest Ophthalmol Vis Sci. 61:19. DOI: 10.1167/iovs.61.8.19. PMID: 32667999. PMCID: PMC7425707. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088157869&origin=inward.
Article31. Han Z, Chen Y, Zhang Y, Wei A, Zhou J, Li Q, Guo L. 2017; MiR-21/PTEN axis promotes skin wound healing by dendritic cells enhancement. J Cell Biochem. 118:3511–3519. DOI: 10.1002/jcb.26026. PMID: 28374893. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026916957&origin=inward.
Article32. Wei F, Wang A, Wang Q, Han W, Rong R, Wang L, Liu S, Zhang Y, Dong C, Li Y. 2020; Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging (Albany NY). 12:12002–12018. DOI: 10.18632/aging.103366. PMID: 32570219. PMCID: PMC7343472. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85087344841&origin=inward.
Article33. Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z, Wu X, Huang Y, Gao B, Wang H, Wu Z, Qiu G. 2020; Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomedicine. 15:7979–7993. DOI: 10.2147/IJN.S275650. PMID: 33116513. PMCID: PMC7585514. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092687407&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-182-5p Mediated by Exosomes Derived From Bone Marrow Mesenchymal Stem Cell Attenuates Inflammatory Responses by Targeting TLR4 in a Mouse Model of Myocardial Infraction
- Exosomes from Tension Force-Applied Periodontal Ligament Cells Promote Mesenchymal Stem Cell Recruitment by Altering microRNA Profiles
- A Glimpse of Urine Stromal Cells-Derived Exosomes Containing Deleted in Malignant Brain Tumors 1: A Critical Factor in Wound Healing
- Integrin β1 in Adipose-Derived Stem Cells Accelerates Wound Healing via Activating PI3K/AKT Pathway
- miR-450a-5p Eliminates MGO-Induced Insulin Resistance via Targeting CREB