Ann Rehabil Med.
2022 Oct;46(5):228-236. 10.5535/arm.22040.
Low-Frequency Repetitive Transcranial Magnetic Stimulation in the Early Subacute Phase of Stroke Enhances Angiogenic Mechanisms in Rats
- Affiliations
-
- 1Department of Physical and Rehabilitation Medicine, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Korea
- 2Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2535740
- DOI: http://doi.org/10.5535/arm.22040
Abstract
Objective
To characterize the repetitive transcranial magnetic stimulation (rTMS) induced changes in angiogenic mechanisms across different brain regions.
Methods
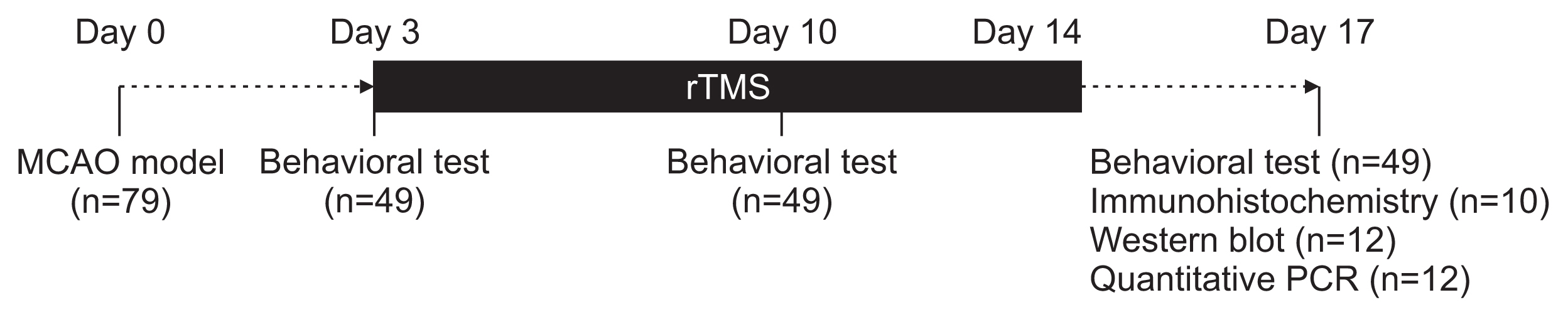
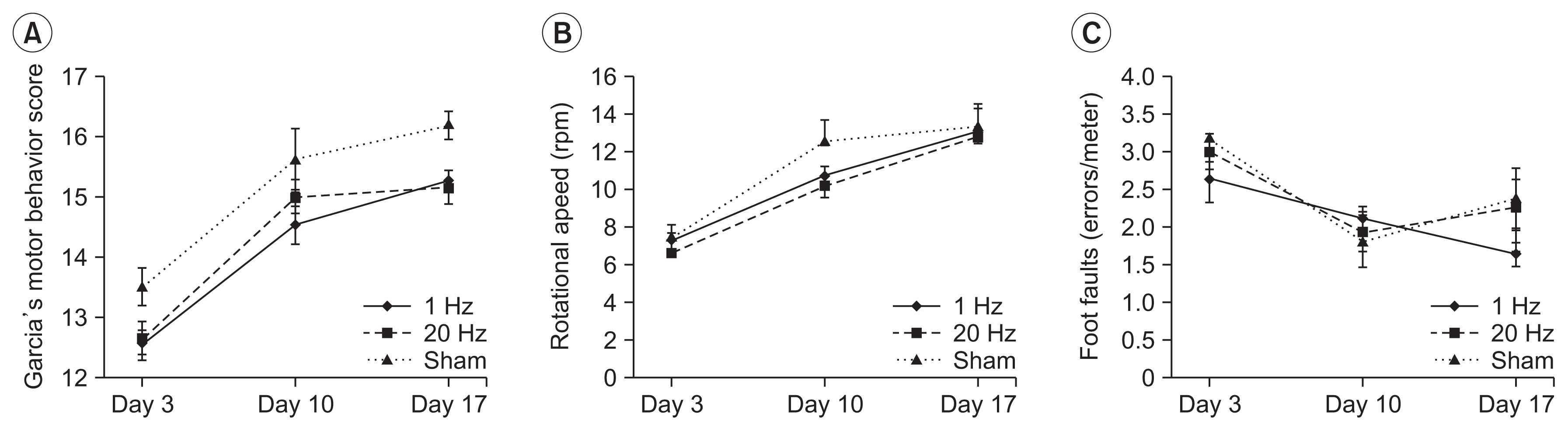
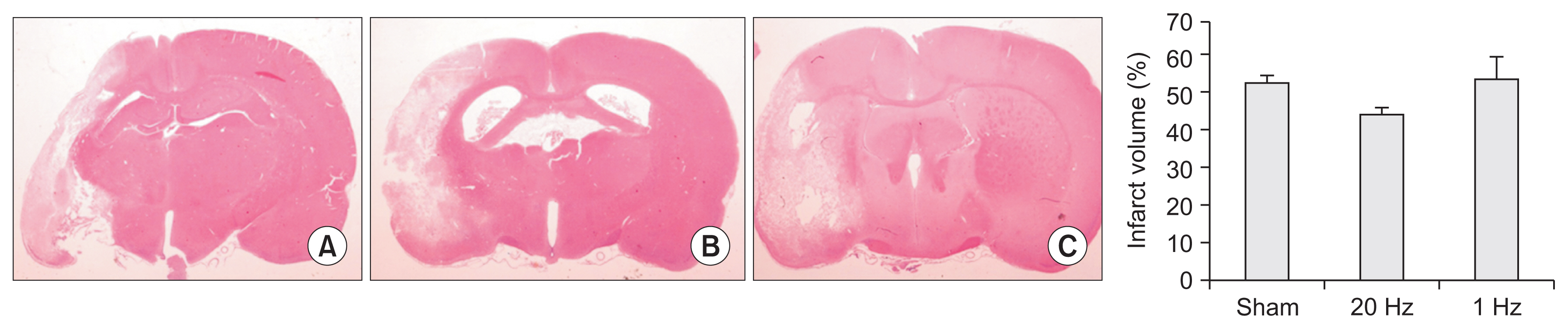
Seventy-nine adult male Sprague-Dawley rats were subjected to a middle cerebral artery occlusion (day 0) and then treated with 1-Hz, 20-Hz, or sham stimulation of their lesioned hemispheres for 2 weeks. The stimulation intensity was set to 100% of the motor threshold. The neurological function was assessed on days 3, 10, and 17. The infarct volume and angiogenesis were measured by histology, immunohistochemistry, Western blot, and real-time polymerase chain reaction (PCR) assays. Brain tissue was harvested from the ischemic core (IC), ischemic border zone (BZ), and contralateral homologous cortex (CH).
Results
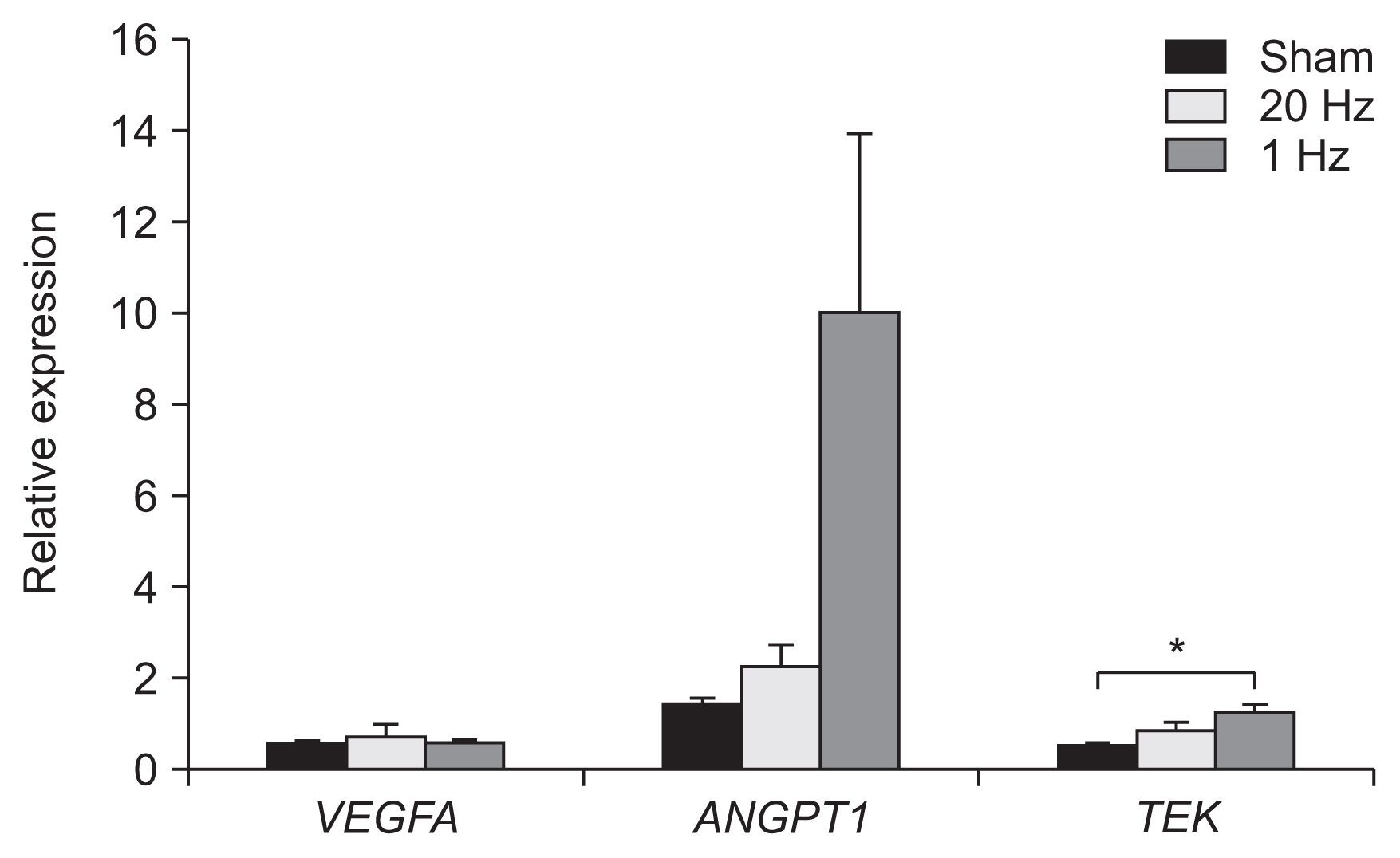
Optical density of angiopoietin1 and synaptophysin in the IC was significantly greater in the low-frequency group than in the sham group (p=0.03 and p=0.03, respectively). The 1-Hz rTMS significantly increased the level of Akt phosphorylation in the BZ (p<0.05 vs. 20 Hz). Endothelial nitric oxide synthase phosphorylation was increased in the IC (p<0.05 vs. 20 Hz), BZ (p<0.05 vs. 20 Hz), and CH (p<0.05 vs. 20 Hz and p<0.05 vs. sham). Real-time PCR demonstrated that low-frequency stimulation significantly increased the transcriptional activity of the TIE2 gene in the IC (p<0.05).
Conclusion
Low-frequency rTMS of the ipsilesional hemisphere in the early subacute phase of stroke promotes the expression of angiogenic factors and related genes in the brain, particularly in the injured area.
Keyword
Figure
Reference
-
1. Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004; 75:612–6.
Article2. Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006; 122:22–7.
Article3. O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007; 62:1208–16.
Article4. Brighina F, Giglia G, Scalia S, Francolini M, Palermo A, Fierro B. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. Exp Brain Res. 2005; 161:34–8.
Article5. Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS). Neurophysiol Clin. 2006; 36:105–15.6. Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005; 65:466–8.
Article7. Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006; 37:2115–22.
Article8. Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994; 117(Pt 4):847–58.
Article9. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007; 8:559–67.
Article10. Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007; 176:603–15.
Article11. Keck ME, Sillaber I, Ebner K, Welt T, Toschi N, Kaehler ST, et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur J Neurosci. 2000; 12:3713–20.
Article12. Post A, Muller MB, Engelmann M, Keck ME. Repetitive transcranial magnetic stimulation in rats: evidence for a neuroprotective effect in vitro and in vivo. Eur J Neurosci. 1999; 11:3247–54.
Article13. Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007; 317:1918–21.
Article14. Ikeda T, Kobayashi S, Morimoto C. Effects of repetitive transcranial magnetic stimulation on ER stressrelated genes and glutamate, γ-aminobutyric acid and glycine transporter genes in mouse brain. Biochem Biophys Rep. 2018; 17:10–6.
Article15. Ljubisavljevic MR, Javid A, Oommen J, Parekh K, Nagelkerke N, Shehab S, et al. The effects of different repetitive transcranial magnetic stimulation (rTMS) protocols on cortical gene expression in a rat model of cerebral ischemic-reperfusion injury. PLoS One. 2015; 10:e0139892.
Article16. Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002; 22:379–92.
Article17. Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012; 43:2270–4.18. Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007; 27:564–74.
Article19. Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000; 156:965–76.
Article20. Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001; 32:2179–84.
Article21. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994; 25:1794–8.
Article22. Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007; 62:49–58.
Article23. Caglayan AB, Beker MC, Caglayan B, Yalcin E, Caglayan A, Yulug B, et al. Acute and post-acute neuromodulation induces stroke recovery by promoting survival signaling, neurogenesis, and pyramidal tract plasticity. Front Cell Neurosci. 2019; 13:144.
Article24. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20:84–91.
Article25. Beom J, Lee JC, Paeng JC, Han TR, Bang MS, Oh BM. Repetitive transcranial magnetic stimulation to the unilateral hemisphere of rat brain. J Vis Exp. 2016; (116):54217.
Article26. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995; 26:627–35.
Article27. Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006; 98:1014–23.
Article28. Seo HG, Kim DY, Park HW, Lee SU, Park SH. Early motor balance and coordination training increased synaptophysin in subcortical regions of the ischemic rat brain. J Korean Med Sci. 2010; 25:1638–45.
Article29. Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006; 5:512–8.
Article30. Teppo J, Vaikkinen A, Stratoulias V, Matlik K, Anttila JE, Smolander OP, et al. Molecular profile of the rat peri-infarct region four days after stroke: study with MANF. Exp Neurol. 2020; 329:113288.
Article31. Yu J, de Muinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005; 102:10999–1004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Effects of Repetitive Transcranial Magnetic Stimulation with High- or Low-frequency on Visuospatial Neglect in Stroke Patients
- Effect of Low-Frequency rTMS and NMES on Subacute Unilateral Hemispheric Stroke With Dysphagia
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation
- Early Augmentation Response with Low-frequency Repetitive Transcranial Magnetic Stimulation in Treatment Resistant Depression