J Korean Assoc Oral Maxillofac Surg.
2022 Oct;48(5):249-258. 10.5125/jkaoms.2022.48.5.249.
Prognosis of tongue squamous cell carcinoma associated with individual surgical margin and pathological features
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Seoul National University, Seoul, Korea
- 2Department of Oral Pathology, Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea
- KMID: 2534804
- DOI: http://doi.org/10.5125/jkaoms.2022.48.5.249
Abstract
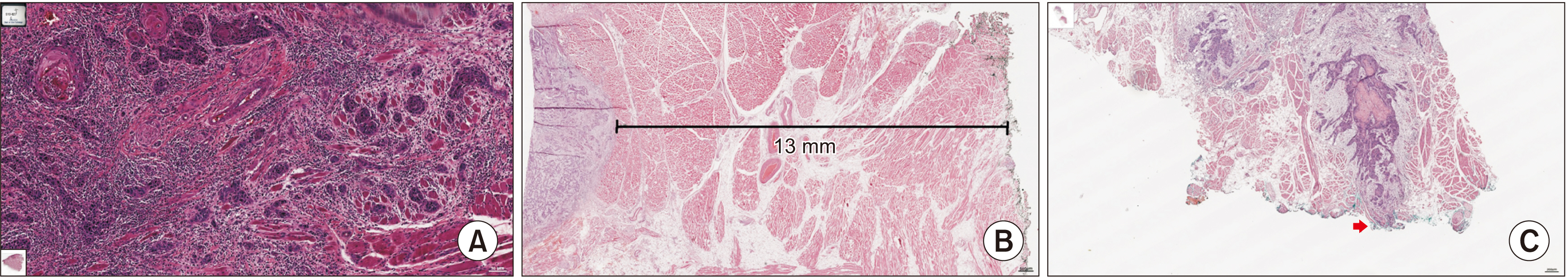
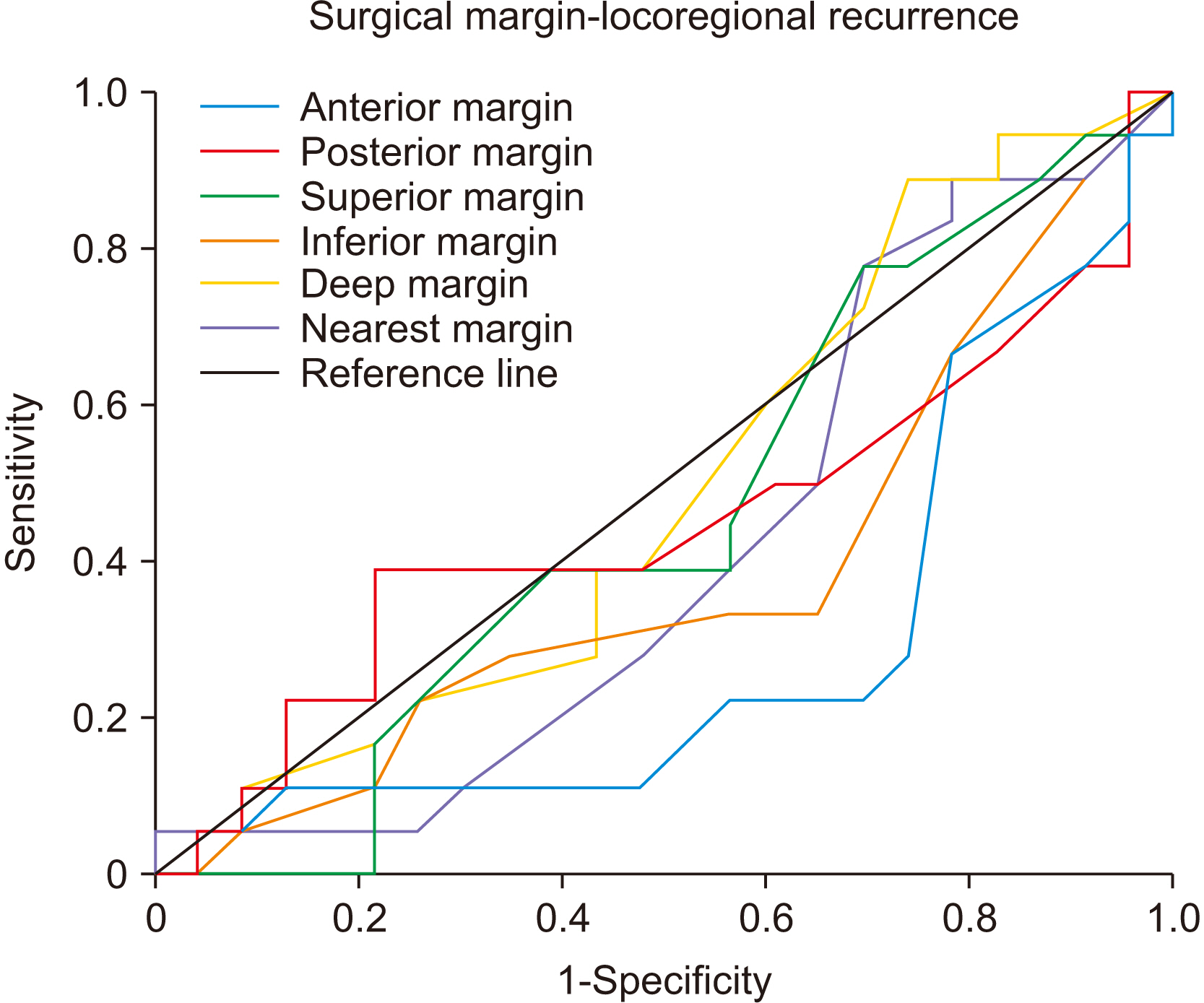
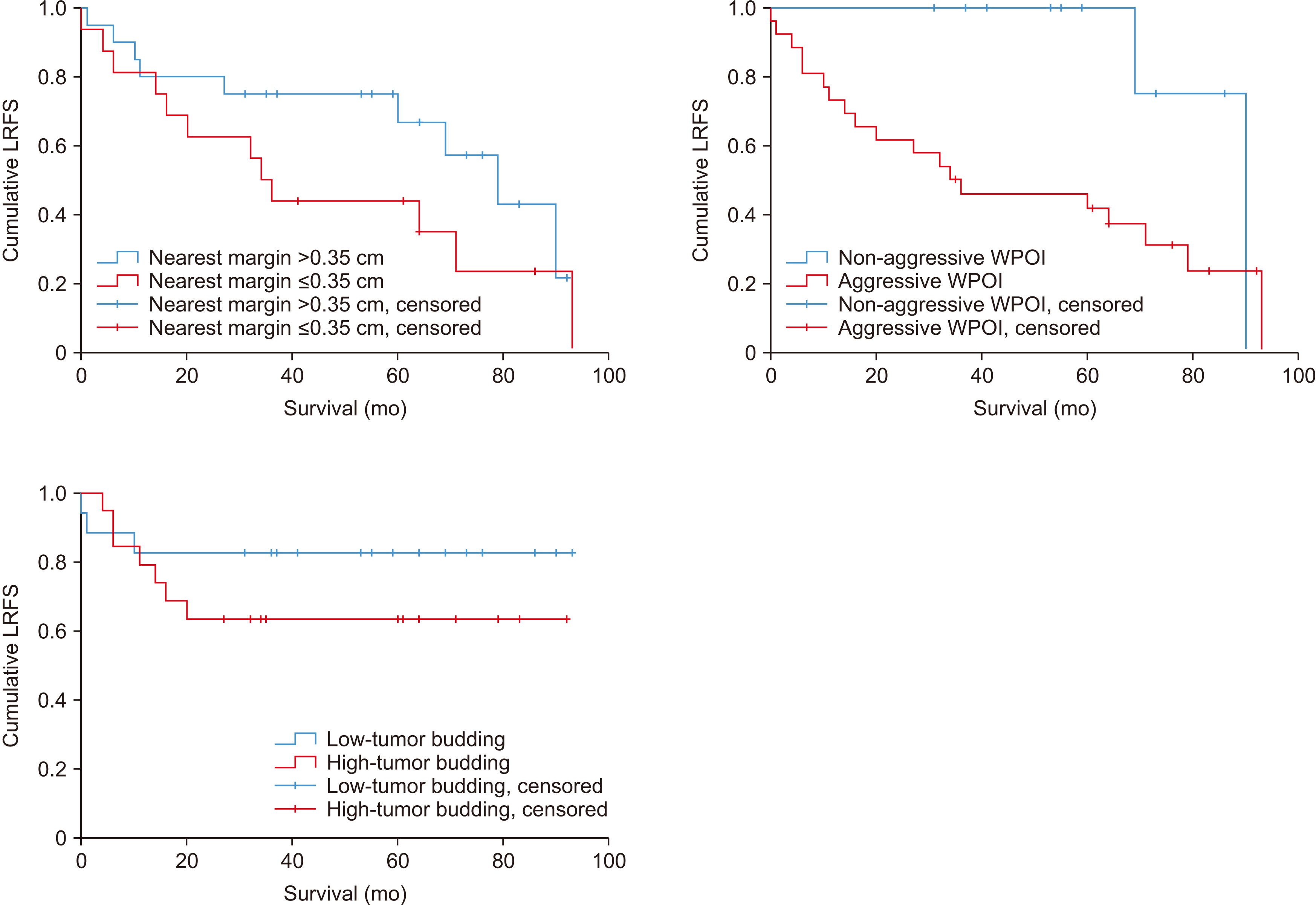
- The specific muscular structure of the tongue greatly affects margin shrinkage and tumor invasion, making the optimal surgical margin controversial. This study investigated surgical margin correlated prognosis of TSCC (tongue squamous cell carcinoma) according to margin location and its value, and the histopathologic factors which are suggestive of tumor invasion. And we would like to propose defining of the surgical margin for TSCC via prognosis according to location and margin values. We reviewed 45 patients diagnosed with TSCC who visited Seoul National University Dental Hospital (SNUDH) (Seoul, Republic of Korea) from 2010 to 2019, who were managed by a single surgical team. Patient clinical and pathological data of patients were retrospectively reviewed, and in 36 out of 45 patients, the pathologic parameters including the worst pattern of invasion (WPOI) and tumor budding were investigated via diagnostic histopathology slide reading. When standardized with as 0.25 cm anterior margins, as 0.35 cm deep margin, there was no significant difference in disease specific survival (DSS) or loco-regional recurrence-free survival (LRFS). Additionally, there was a non-significant difference in DSS and LRFS at the nearest margin of 0.35 cm (PDSS =0.276, PLRFS =0.162). Aggressive WPOI and high tumor budding showed lower survival and recurrence-free survival, and there were significant differences in close margin and involved margin frequencies. In TSCC, the value and location of the surgical margin did not have a significant relationship with prognosis, but WPOI and tumor budding suggesting the pattern of muscle invasion affected survival and recurrence-free survival. WPOI and tumor budding should be considered when setting an optimal surgical margin.
Keyword
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. 2016; Cancer statistics, 2016. CA Cancer J Clin. 66:7–30. https://doi.org/10.3322/caac.21332. DOI: 10.3322/caac.21332. PMID: 26742998.
Article2. Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, et al. 2014; Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 36:811–8. https://doi.org/10.1002/hed.23380. DOI: 10.1002/hed.23380. PMID: 23696499. PMCID: PMC4229066.
Article3. Calabrese L, Bizzoca ME, Grigolato R, Maffini FA, Tagliabue M, Negro R, et al. 2020; From bench to bedside in tongue muscle cancer invasion and back again: gross anatomy, microanatomy, surgical treatments and basic research. Life (Basel). 10:197. https://doi.org/10.3390/life10090197. DOI: 10.3390/life10090197. PMID: 32932638. PMCID: PMC7554763.
Article4. Kamat M, Rai BD, Puranik RS, Datar UV. 2019; A comprehensive review of surgical margin in oral squamous cell carcinoma highlighting the significance of tumor-free surgical margins. J Cancer Res Ther. 15:449–54. https://doi.org/10.4103/jcrt.JCRT_273_17. DOI: 10.4103/jcrt.JCRT_273_17. PMID: 31169203.
Article5. Matte BF, Kumar A, Placone JK, Zanella VG, Martins MD, Engler AJ, et al. 2019; Matrix stiffness mechanically conditions EMT and migratory behavior of oral squamous cell carcinoma. J Cell Sci. 132:jcs224360. https://doi.org/10.1242/jcs.224360. DOI: 10.1242/jcs.224360. PMID: 30559248. PMCID: PMC6340137.
Article6. Li Y, Bai S, Carroll W, Dayan D, Dort JC, Heller K, et al. 2013; Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 7:211–23. https://doi.org/10.1007/s12105-012-0412-1. DOI: 10.1007/s12105-012-0412-1. PMID: 23250819. PMCID: PMC3738758.
Article7. Chatterjee D, Bansal V, Malik V, Bhagat R, Punia RS, Handa U, et al. 2019; Tumor budding and worse pattern of invasion can predict nodal metastasis in oral cancers and associated with poor survival in early-stage tumors. Ear Nose Throat J. 98:E112–9. https://doi.org/10.1177/0145561319848669. DOI: 10.1177/0145561319848669. PMID: 31072197.
Article8. Almangush A, Bello IO, Coletta RD, Mäkitie AA, Mäkinen LK, Kauppila JH, et al. 2015; For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch. 467:39–46. https://doi.org/10.1007/s00428-015-1758-z. DOI: 10.1007/s00428-015-1758-z. PMID: 25838076.
Article9. Dillon JK, Brown CB, McDonald TM, Ludwig DC, Clark PJ, Leroux BG, et al. 2015; How does the close surgical margin impact recurrence and survival when treating oral squamous cell carcinoma? J Oral Maxillofac Surg. 73:1182–8. https://doi.org/10.1016/j.joms.2014.12.014. DOI: 10.1016/j.joms.2014.12.014. PMID: 25795179.
Article10. Alicandri-Ciufelli M, Bonali M, Piccinini A, Marra L, Ghidini A, Cunsolo EM, et al. 2013; Surgical margins in head and neck squamous cell carcinoma: what is 'close'? Eur Arch Otorhinolaryngol. 270:2603–9. https://doi.org/10.1007/s00405-012-2317-8. DOI: 10.1007/s00405-012-2317-8. PMID: 23271033.
Article11. Bungum A, Jensen JS, Jakobsen KK, Christensen A, Grønhøj C, von Buchwald C. 2020; Impact of surgical resection margins less than 5 mm in oral cavity squamous cell carcinoma: a systematic review. Acta Otolaryngol. 140:869–75. https://doi.org/10.1080/00016489.2020.1773532. DOI: 10.1080/00016489.2020.1773532. PMID: 32564643.
Article12. Zanoni DK, Migliacci JC, Xu B, Katabi N, Montero PH, Ganly I, et al. 2017; A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 143:555–60. https://doi.org/10.1001/jamaoto.2016.4238. DOI: 10.1001/jamaoto.2016.4238. PMID: 28278337. PMCID: PMC5473778.
Article13. Singh A, Mishra A, Singhvi H, Sharin F, Bal M, Laskar SG, et al. 2020; Optimum surgical margins in squamous cell carcinoma of the oral tongue: is the current definition adequate? Oral Oncol. 111:104938. https://doi.org/10.1016/j.oraloncology.2020.104938. DOI: 10.1016/j.oraloncology.2020.104938. PMID: 32739791.
Article14. Lee DY, Kang SH, Kim JH, Kim MS, Oh KH, Woo JS, et al. 2018; Survival and recurrence of resectable tongue cancer: resection margin cutoff value by T classification. Head Neck. 40:283–91. https://doi.org/10.1002/hed.24944. DOI: 10.1002/hed.24944. PMID: 28960654.
Article15. Jadhav KB, Gupta N. 2013; Clinicopathological prognostic implicators of oral squamous cell carcinoma: need to understand and revise. N Am J Med Sci. 5:671–9. https://doi.org/10.4103/1947-2714.123239. DOI: 10.4103/1947-2714.123239. PMID: 24404549. PMCID: PMC3877528.
Article16. Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA, et al. 2011; Compartmental tongue surgery: long term oncologic results in the treatment of tongue cancer. Oral Oncol. 47:174–9. https://doi.org/10.1016/j.oraloncology.2010.12.006. DOI: 10.1016/j.oraloncology.2010.12.006. PMID: 21257337.
Article17. Pu Y, Ding L, Wang Y, Wang Y, Chen S, Huang X, et al. 2021; Biopsy pattern of invasion type to determine the surgical approach in early-stage oral squamous cell carcinoma. Virchows Arch. 479:109–19. https://doi.org/10.1007/s00428-020-03008-y. DOI: 10.1007/s00428-020-03008-y. PMID: 33438091.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Basaloid Squamous Cell Carcinoma Occurring in the Mobile Tongue
- Locoregional Recurrence of a Tongue Cancer Patient with 10 Year Follow-up
- Treatment of Adeno-Squamous Cell Carcinoma at the Posterior Tongue via Median Glossotomy Approach
- Glossopharyngeal Neuralgia Secondary to Tongue Squamous Cell Carcinoma
- Clinical Outcome of Squamous Cell Carcinoma of the Tongue in Young Patients: A Stage-Matched Comparative Analysis