Korean J Physiol Pharmacol.
2022 Nov;26(6):479-499. 10.4196/kjpp.2022.26.6.479.
Cell-cell contacts via N-cadherin induce a regulatory renin secretory phenotype in As4.1 cells
- Affiliations
-
- 1Department of Physiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea
- 3Department of Internal Medicine, The Catholic University of Korea, Seoul St. Mary’s Hospital, Seoul 06591, Korea
- 4Department of Physiology, Jeonbuk National University Medical School, Jeonju 54907, Korea
- KMID: 2534524
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.479
Abstract
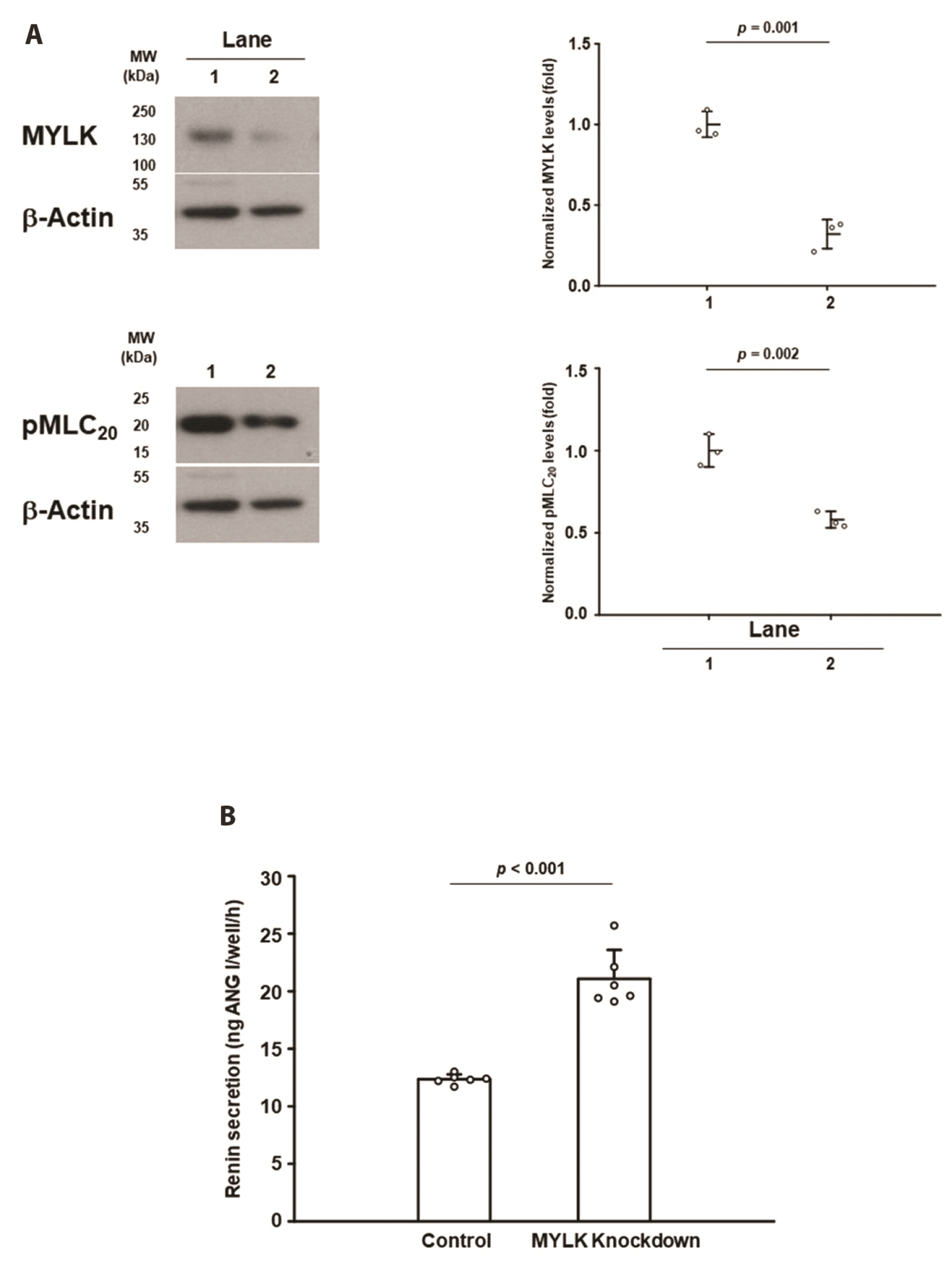
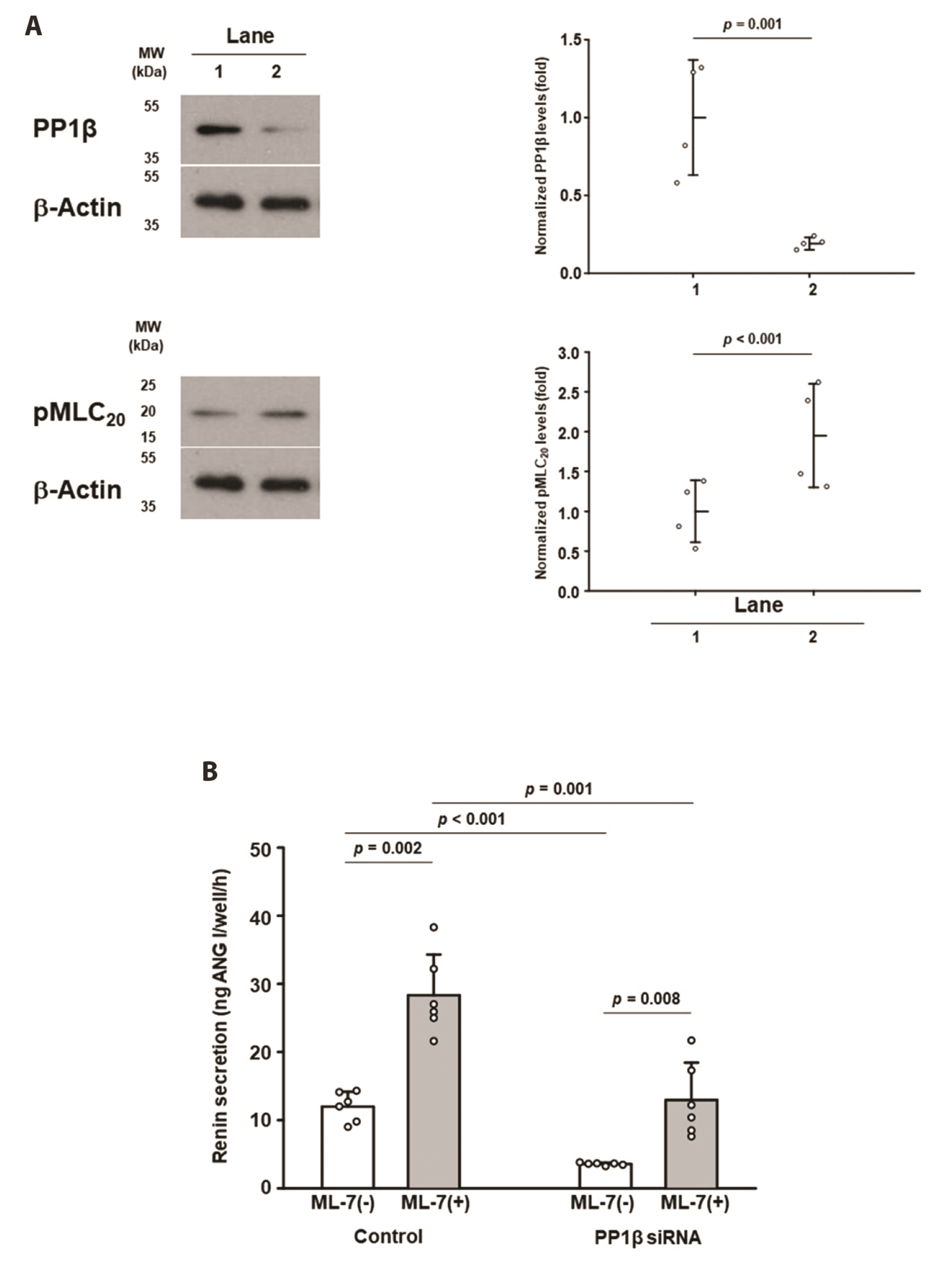
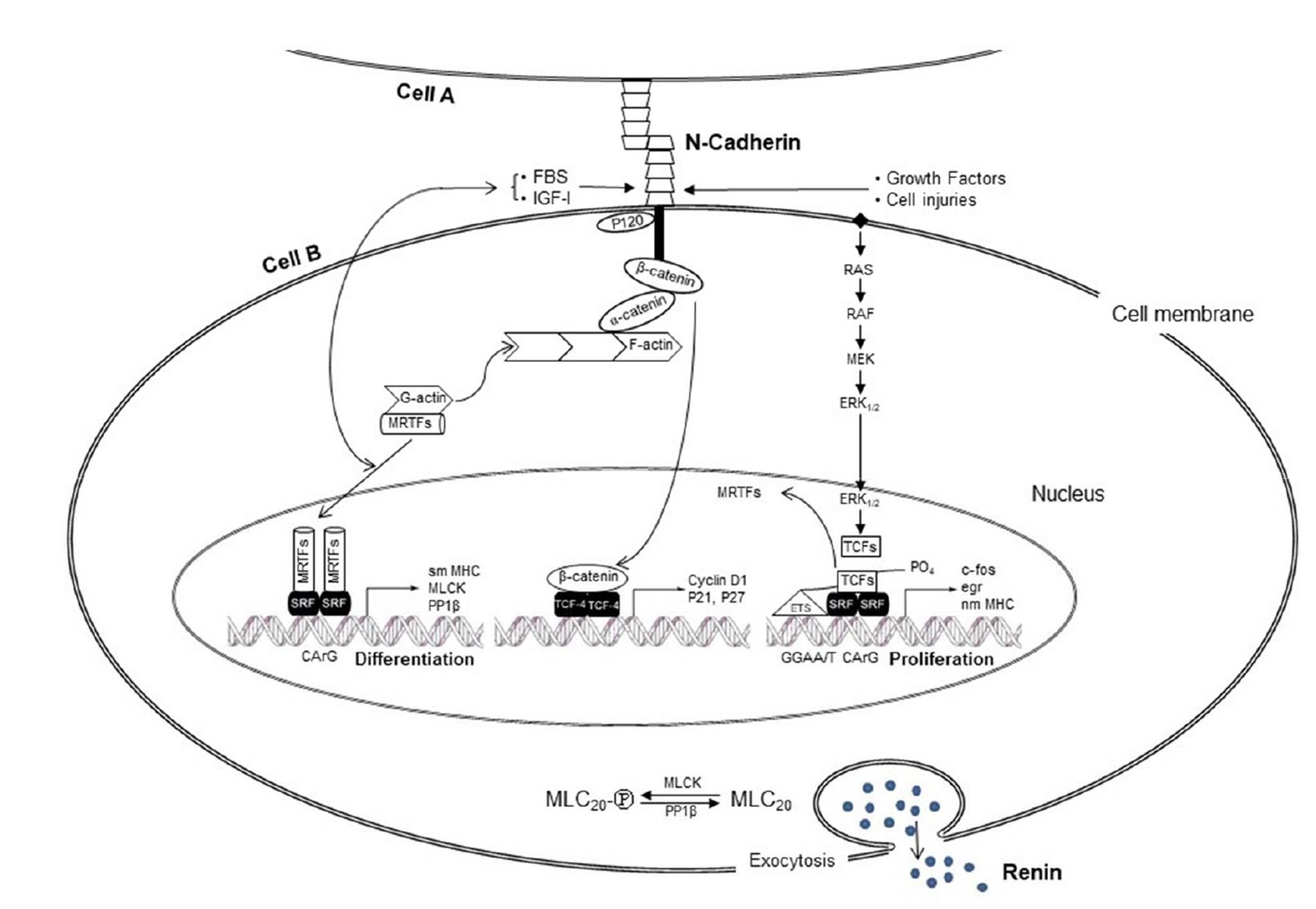
- The lack of a clonal renin-secreting cell line has greatly hindered the investigation of the regulatory mechanisms of renin secretion at the cellular, biochemical, and molecular levels. In the present study, we investigated whether it was possible to induce phenotypic switching of the renin-expressing clonal cell line As4.1 from constitutive inactive renin secretion to regulated active renin secretion. When grown to postconfluence for at least two days in media containing fetal bovine serum or insulin-like growth factor-1, the formation of cell-cell contacts via N-cadherin triggered downstream cellular signaling cascades and activated smooth muscle-specific genes, culminating in phenotypic switching to a regulated active renin secretion phenotype, including responding to the key stimuli of active renin secretion. With the use of phenotype-switched As4.1 cells, we provide the first evidence that active renin secretion via exocytosis is regulated by phosphorylation/dephosphorylation of the 20 kDa myosin light chain. The molecular mechanism of phenotypic switching in As4.1 cells described here could serve as a working model for full phenotypic modulation of other secretory cell lines with incomplete phenotypes.
Keyword
Figure
Reference
-
1. Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH. 1982; The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology. 111:86–94. DOI: 10.1210/endo-111-1-86. PMID: 6123433. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0020164058&origin=inward.
Article2. Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. 1999; Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 48:1402–1408. DOI: 10.2337/diabetes.48.7.1402. PMID: 10389845. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032992378&origin=inward.
Article3. Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger JC. 2003; Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology. 144:1368–1379. DOI: 10.1210/en.2002-220916. PMID: 12639920. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037390423&origin=inward.
Article4. Hackenthal E, Paul M, Ganten D, Taugner R. 1990; Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 70:1067–1116. DOI: 10.1152/physrev.1990.70.4.1067. PMID: 2217555. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0025005906&origin=inward.
Article5. Cabandugama PK, Gardner MJ, Sowers JR. 2017; The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin North Am. 101:129–137. DOI: 10.1016/j.mcna.2016.08.009. PMID: 27884224. PMCID: PMC5125542. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84996743333&origin=inward.6. Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. 2011; Genes that confer the identity of the renin cell. J Am Soc Nephrol. 22:2213–2225. DOI: 10.1681/ASN.2011040401. PMID: 22034642. PMCID: PMC3279933. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=82655173696&origin=inward.
Article7. Della Bruna R, Kurtz A. 1995; Juxtaglomerular cells in culture. Exp Nephrol. 3:219–222. PMID: 8590034. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029101966&origin=inward.8. Galen FX, Devaux C, Houot AM, Menard J, Corvol P, Corvol MT, Gubler MC, Mounier F, Camilleri JP. 1984; Renin biosynthesis by human tumoral juxtaglomerular cells. Evidences for a renin precursor. J Clin Invest. 73:1144–1155. DOI: 10.1172/JCI111300. PMID: 6323535. PMCID: PMC425128. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0021270652&origin=inward.
Article9. Pinet F, Corvol MT, Dench F, Bourguignon J, Feunteun J, Menard J, Corvol P. 1985; Isolation of renin-producing human cells by transfection with three simian virus 40 mutants. Proc Natl Acad Sci U S A. 82:8503–8507. DOI: 10.1073/pnas.82.24.8503. PMID: 3001706. PMCID: PMC390944. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0022371679&origin=inward.
Article10. Sigmund CD, Okuyama K, Ingelfinger J, Jones CA, Mullins JJ, Kane C, Kim U, Wu CZ, Kenny L, Rustum Y, Dzau VJ, Gross KW. 1990; Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem. 265:19916–19922. DOI: 10.1016/S0021-9258(17)45460-3. PMID: 2174057.
Article11. Grünberger C, Obermayer B, Klar J, Kurtz A, Schweda F. 2006; The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res. 99:1197–1206. DOI: 10.1161/01.RES.0000251057.35537.d3. PMID: 17068292. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33751335626&origin=inward.12. Jensen BL, Lehle U, Müller M, Wagner C, Kurtz A. 1998; Interleukin-1 inhibits renin gene expression in As4.1 cells but not in native juxtaglomerular cells. Pflugers Arch. 436:673–678. DOI: 10.1007/s004240050688. PMID: 9716699. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031693541&origin=inward.
Article13. Jones CA, Petrovic N, Novak EK, Swank RT, Sigmund CD, Gross KW. 1997; Biosynthesis of renin in mouse kidney tumor As4.1 cells. Eur J Biochem. 243:181–190. DOI: 10.1111/j.1432-1033.1997.0181a.x. PMID: 9030738. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031019662&origin=inward.
Article14. Klar J, Sandner P, Müller MW, Kurtz A. 2002; Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 444:335–344. DOI: 10.1007/s00424-002-0818-9. PMID: 12111241. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036448341&origin=inward.
Article15. Laframboise M, Reudelhuber TL, Jutras I, Brechler V, Seidah NG, Day R, Gross KW, Deschepper CF. 1997; Prorenin activation and prohormone convertases in the mouse As4.1 cell line. Kidney Int. 51:104–109. DOI: 10.1038/ki.1997.13. PMID: 8995723. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031042265&origin=inward.
Article16. Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH. 2004; Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol. 287:F329–F335. DOI: 10.1152/ajprenal.00420.2003. PMID: 15082450. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=3242716881&origin=inward.
Article17. Beierwaltes WH. 2012; Hydrogen sulfide, renin, and regulating the second messenger cAMP. Focus on "Hydrogen sulfide regulates cAMP homeostasis and renin degranulation in As4.1 and rat renin-rich kidney cell.". Am J Physiol Cell Physiol. 302:C21–C23. DOI: 10.1152/ajpcell.00375.2011. PMID: 21998138. PMCID: PMC3328909. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=83455223699&origin=inward.18. Chamley-Campbell JH, Campbell GR. 1981; What controls smooth muscle phenotype? Atherosclerosis. 40:347–357. https://doi.org/10.1016/0021-9150(81)90145-3. DOI: 10.1016/0021-9150(81)90145-3. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019458817&origin=inward.
Article19. Owens GK, Kumar MS, Wamhoff BR. 2004; Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 84:767–801. DOI: 10.1152/physrev.00041.2003. PMID: 15269336. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=3042588831&origin=inward.
Article20. Kawamoto S, Adelstein RS. 1987; Characterization of myosin heavy chains in cultured aorta smooth muscle cells. A comparative study. J Biol Chem. 262:7282–7288. DOI: 10.1016/S0021-9258(18)48234-8. PMID: 2438275.
Article21. Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. 2004; The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 286:C507–C517. DOI: 10.1152/ajpcell.00201.2003. PMID: 14592809. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=1442325390&origin=inward.
Article22. George SJ, Beeching CA. 2006; Cadherin:catenin complex: a novel regulator of vascular smooth muscle cell behaviour. Atherosclerosis. 188:1–11. DOI: 10.1016/j.atherosclerosis.2005.12.017. PMID: 16438974. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746478732&origin=inward.
Article23. Quasnichka H, Slater SC, Beeching CA, Boehm M, Sala-Newby GB, George SJ. 2006; Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ Res. 99:1329–1337. DOI: 10.1161/01.RES.0000253533.65446.33. PMID: 17122440. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33845620229&origin=inward.
Article24. Uglow EB, Slater S, Sala-Newby GB, Aguilera-Garcia CM, Angelini GD, Newby AC, George SJ. 2003; Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ Res. 92:1314–1321. DOI: 10.1161/01.RES.0000079027.44309.53. PMID: 12775583. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038801626&origin=inward.
Article25. Gurney EG, Gurney T Jr. 1979; Density dependent inhibition of both growth and T-antigen expression in revertants isolated from simian virus 40-transformed mouse SVT2 cells. J Virol. 32:667–671. DOI: 10.1128/jvi.32.2.667-671.1979. PMID: 228083. PMCID: PMC353598. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0018538248&origin=inward.
Article26. Ganot N, Meker S, Reytman L, Tzubery A, Tshuva EY. 2013; Anticancer metal complexes: synthesis and cytotoxicity evaluation by the MTT assay. J Vis Exp. (81):e50767. DOI: 10.3791/50767. PMID: 24300943. PMCID: PMC3989495. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903080854&origin=inward.
Article27. Park CS, Lee HS, Chang SH, Honeyman TW, Hong CD. 1996; Inhibitory effect of Ca2+ on renin secretion elicited by chemiosmotic stimuli through actomyosin mediation. Am J Physiol. 271(1 Pt 1):C248–C254. DOI: 10.1152/ajpcell.1996.271.1.C248. PMID: 8760053. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030192099&origin=inward.28. Ludowyke RI, Peleg I, Beaven MA, Adelstein RS. 1989; Antigen-induced secretion of histamine and the phosphorylation of myosin by protein kinase C in rat basophilic leukemia cells. J Biol Chem. 264:12492–12501. DOI: 10.1016/S0021-9258(18)63885-2. PMID: 2473073.
Article29. Bradford MM. 1976; A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. DOI: 10.1016/0003-2697(76)90527-3. PMID: 942051. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0017184389&origin=inward.
Article30. Harada K. 1954; Histochemical studies of the juxta glomerular apparatus. Rev Belg Pathol Med Exp. 23:311–320. PMID: 14372543. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77049215216&origin=inward.31. Knudsen KA, Frankowski C, Johnson KR, Wheelock MJ. 1998; A role for cadherins in cellular signaling and differentiation. J Cell Biochem. 72(S30-S31):168–176. DOI: 10.1002/(SICI)1097-4644(1998)72:30/31+<168::AID-JCB21>3.0.CO;2-V. PMID: 29345834.
Article32. Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. 1987; Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 262:7796–7801. DOI: 10.1016/S0021-9258(18)47638-7. PMID: 3108259.
Article33. Park CS, Kim MH, Leem CH, Jang YJ, Kim HW, Kim HS, et al. 1998; Inhibitory effect of calyculin A, a Ser/Thr protein phosphatase type I inhibitor, on renin secretion. Am J Physiol. 275:F664–F670. DOI: 10.1152/ajprenal.1998.275.5.F664. PMID: 9815125. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17344364413&origin=inward.
Article34. Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ. 2007; Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 282:36112–36120. DOI: 10.1074/jbc.M703914200. PMID: 17908691. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=37249046846&origin=inward.
Article35. Park CS, Chang SH, Lee HS, Kim SH, Chang JW, Hong CD. 1996; Inhibition of renin secretion by Ca2+ through activation of myosin light chain kinase. Am J Physiol. 271(1 Pt 1):C242–C247. DOI: 10.1152/ajpcell.1996.271.1.C242. PMID: 8760052. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0043062950&origin=inward.36. Park CS, Sigmon DH, Han DS, Honeyman TW, Fray JC. 1986; Control of renin secretion by Ca2+ and cyclic AMP through two parallel mechanisms. Am J Physiol. 251(3 Pt 2):R531–R536. DOI: 10.1152/ajpregu.1986.251.3.R531. PMID: 3019164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023019413&origin=inward.37. Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D, Hartshorne DJ. 1989; Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 159:871–877. DOI: 10.1016/0006-291X(89)92189-X. PMID: 2539153. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0024517280&origin=inward.
Article38. Kim MH, Kim SH, Kim HS, Chang JW, Hong YS, Kim HW, Park CS. 1998; Regulation of renin secretion through reversible phosphorylation of myosin by myosin light chain kinase and protein phosphatase type 1. J Pharmacol Exp Ther. 285:968–974. PMID: 9618396. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=7144261705&origin=inward.39. Haber E, Koerner T, Page LB, Kliman B, Purnode A. 1969; Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 29:1349–1355. DOI: 10.1210/jcem-29-10-1349. PMID: 4311082. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0014584257&origin=inward.
Article40. Taugner R, Whalley A, Angermüller S, Bührle CP, Hackenthal E. 1985; Are the renin-containing granules of juxtaglomerular epithelioid cells modified lysosomes? Cell Tissue Res. 239:575–587. DOI: 10.1007/BF00219236. PMID: 3886148. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0021999739&origin=inward.
Article41. Faust PL, Kornfeld S, Chirgwin JM. 1985; Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 82:4910–4914. DOI: 10.1073/pnas.82.15.4910. PMID: 3927292. PMCID: PMC390467. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0011288815&origin=inward.
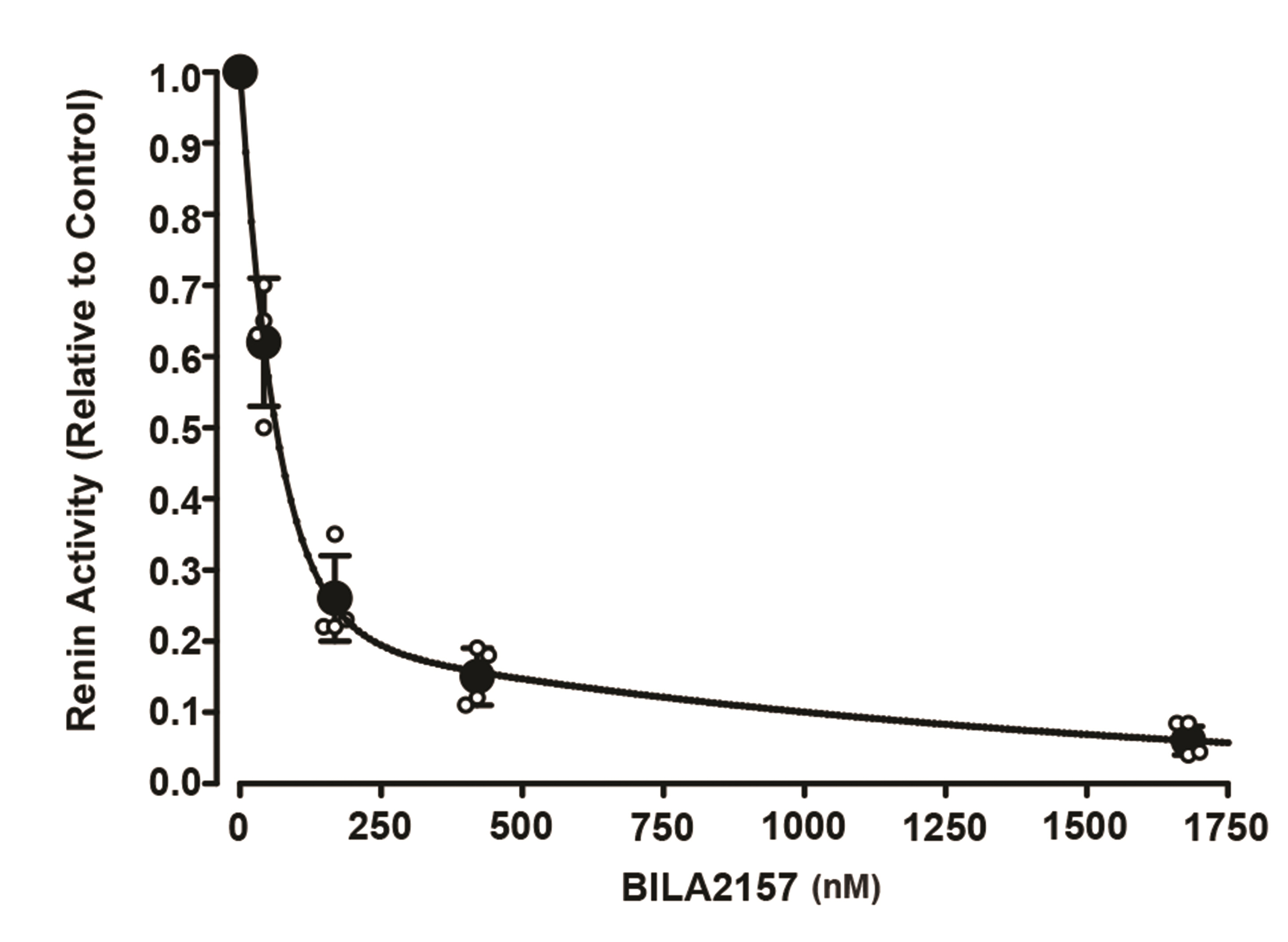
Article42. Beaulieu PL, Gillard J, Bailey M, Beaulieu C, Duceppe JS, Lavallée P, Wernic D. 1999; Practical synthesis of BILA 2157 BS, a potent and orally active renin inhibitor: use of an enzyme-catalyzed hydrolysis for the preparation of homochiral succinic acid derivatives. J Org Chem. 64:6622–6634. DOI: 10.1021/jo990321x. PMID: 11674665. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032885570&origin=inward.
Article43. Park CS, Honeyman TW, Chung ES, Lee JS, Sigmon DH, Fray JC. 1986; Involvement of calmodulin in mediating inhibitory action of intracellular Ca2+ on renin secretion. Am J Physiol. 251(6 Pt 2):F1055–F1062. DOI: 10.1152/ajprenal.1986.251.6.F1055. PMID: 3538904. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023007879&origin=inward.44. Park CS, Malvin RL. 1978; Calcium in the control of renin release. Am J Physiol. 235:F22–F25. DOI: 10.1152/ajprenal.1978.235.1.F22. PMID: 98060. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=18144444810&origin=inward.
Article45. Park CS, Hong CD, Honeyman TW. 1992; Calcium-dependent inhibitory step in control of renin secretion. Am J Physiol. 262(5 Pt 2):F793–F798. DOI: 10.1152/ajprenal.1992.262.5.F793. PMID: 1590424.
Article46. Van Belle H. 1981; R 24 571: a potent inhibitor of calmodulin-activated enzymes. Cell Calcium. 2:483–494. https://doi.org/10.1016/0143-4160(81)90007-5. DOI: 10.1016/0143-4160(81)90007-5. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019718498&origin=inward.
Article47. Seamon KB, Daly JW. 1981; Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 7:201–224. PMID: 6278005. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019811368&origin=inward.48. Miralles F, Posern G, Zaromytidou AI, Treisman R. 2003; Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 113:329–342. DOI: 10.1016/S0092-8674(03)00278-2. PMID: 12732141. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038737042&origin=inward.
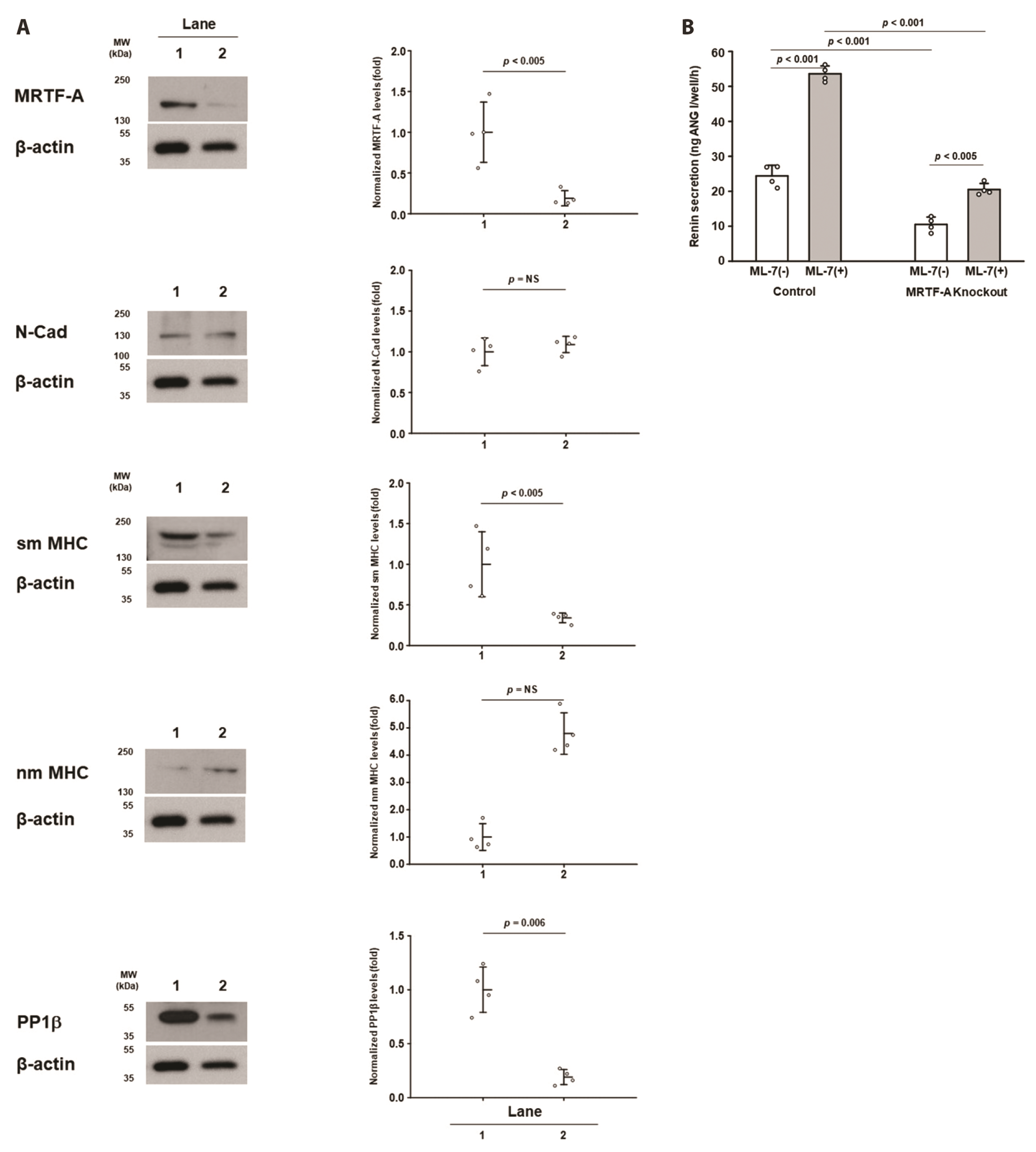
Article49. Wang Z, Wang DZ, Pipes GC, Olson EN. 2003; Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 100:7129–7134. DOI: 10.1073/pnas.1232341100. PMID: 12756293. PMCID: PMC165841. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037795465&origin=inward.
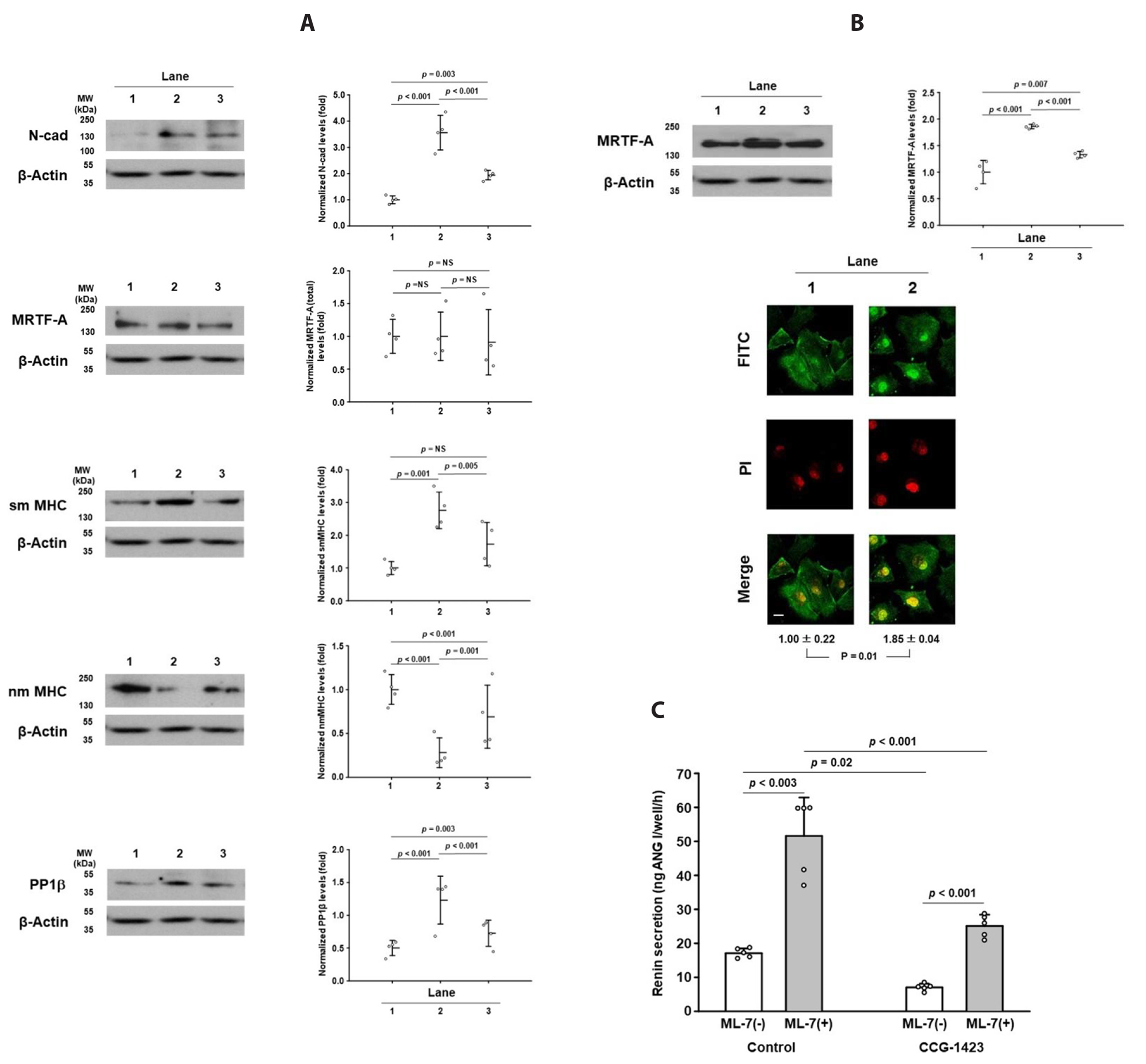
Article50. Evelyn CR, Wade SM, Wang Q, Wu M, Iñiguez-Lluhí JA, Merajver SD, Neubig RR. 2007; CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 6:2249–2260. DOI: 10.1158/1535-7163.MCT-06-0782. PMID: 17699722. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548066554&origin=inward.
Article51. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013; Multiplex genome engineering using CRISPR/Cas systems. Science. 339:819–823. DOI: 10.1126/science.1231143. PMID: 23287718. PMCID: PMC3795411. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84873729095&origin=inward.
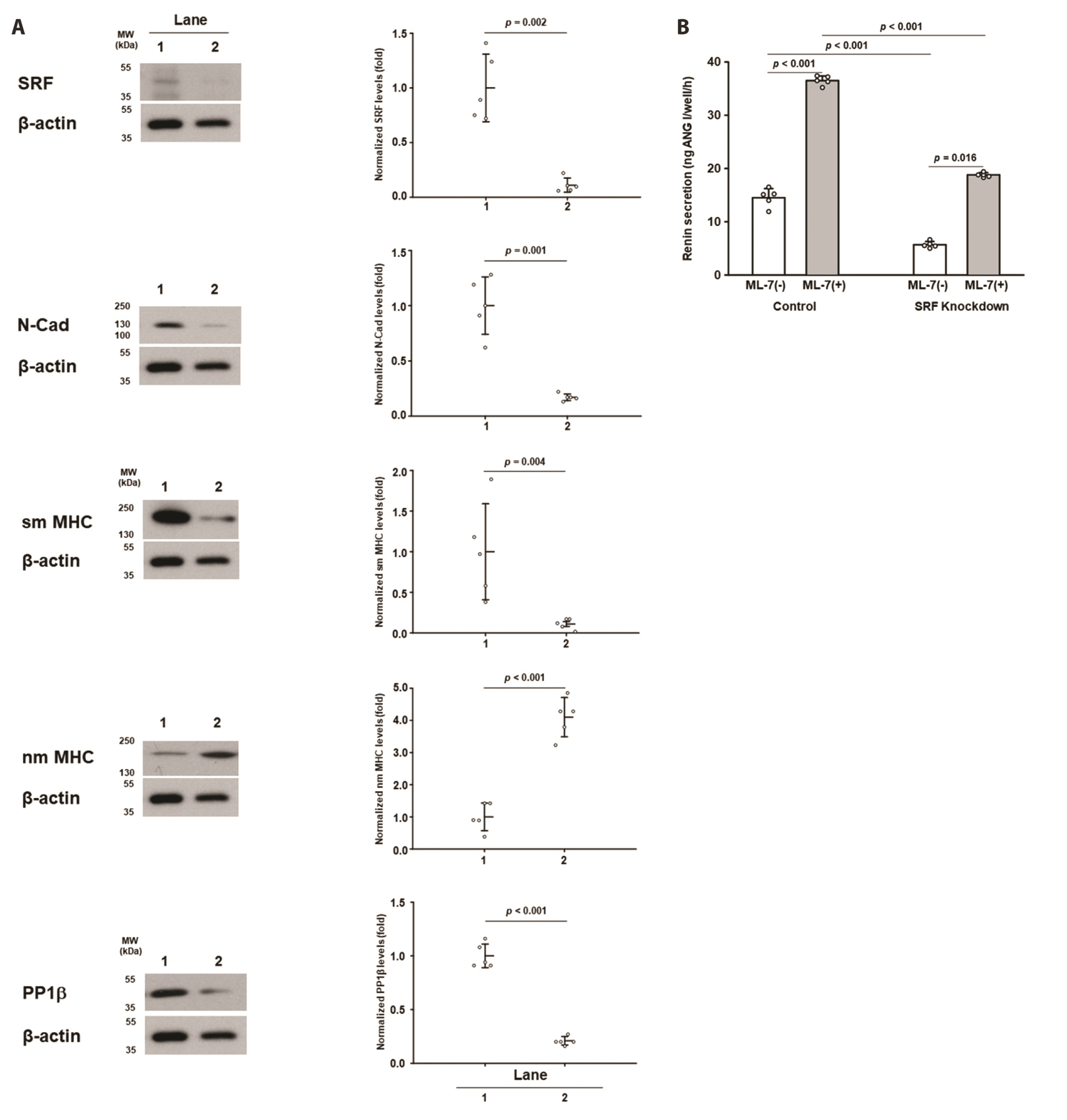
Article52. Miano JM, Long X, Fujiwara K. 2007; Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 292:C70–C81. DOI: 10.1152/ajpcell.00386.2006. PMID: 16928770. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846301667&origin=inward.
Article53. Heemskerk JW, Farndale RW, Sage SO. 1997; Effects of U73122 and U73343 on human platelet calcium signalling and protein tyrosine phosphorylation. Biochim Biophys Acta. 1355:81–88. DOI: 10.1016/S0167-4889(96)00113-9. PMID: 9030204. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031037686&origin=inward.
Article54. Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. 2011; Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension. 57:460–468. DOI: 10.1161/HYPERTENSIONAHA.110.167130. PMID: 21282557. PMCID: PMC3106204. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79953241859&origin=inward.
Article55. Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. 1985; Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 312:1412–1417. DOI: 10.1056/NEJM198505303122202. PMID: 3887168. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0021889169&origin=inward.
Article56. Todaro GJ, Lazar GK, Green H. 1965; The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 66:325–333. DOI: 10.1002/jcp.1030660310. PMID: 5884360. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0013831072&origin=inward.
Article57. Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. 1994; Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 372:570–573. DOI: 10.1038/372570a0. PMID: 7990932. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028172867&origin=inward.
Article58. Posern G, Treisman R. 2006; Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 16:588–596. DOI: 10.1016/j.tcb.2006.09.008. PMID: 17035020. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33750343214&origin=inward.
Article59. Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. 2001; Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 105:851–862. DOI: 10.1016/S0092-8674(01)00404-4. PMID: 11439182. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035967868&origin=inward.
Article60. Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. 2004; Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 428:185–189. DOI: 10.1038/nature02382. PMID: 15014501. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=1642297200&origin=inward.
Article61. Simons M, Rosenberg RD. 1992; Antisense nonmuscle myosin heavy chain and c-myb oligonucleotides suppress smooth muscle cell proliferation in vitro. Circ Res. 70:835–843. DOI: 10.1161/01.RES.70.4.835. PMID: 1551207. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0026536410&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- E-Cadherin Expression and DNA Ploidy Analysis in Invasive Squamous Cell Carcinoma of the Uterine Cervix Comparison with those of CIN
- Cyclooxygenase-2 Expression Is Related to the Epithelial-to-Mesenchymal Transition in Human Colon Cancers
- Regulatory T Cells in B Cell Follicles
- Changes in the Rate of Renin Secretion During Cell Cycle of As 4.1 Cells
- Role of Basal Cell and Secretory Cell in Benign Prostatic Hyperplasia and Prostatic Cancer