Korean J Physiol Pharmacol.
2022 Nov;26(6):439-446. 10.4196/kjpp.2022.26.6.439.
Antitumor effects of valdecoxib on hypopharyngeal squamous carcinoma cells
- Affiliations
-
- 1Department of Pharmacology and Dental Therapeutics, College of Dentistry, Chosun University, Gwangju 61452, Korea
- 2Department of Pharmacy, Thai Binh University of Medicine and Pharmacy, Thai Binh City 06000, Vietnam
- KMID: 2534520
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.439
Abstract
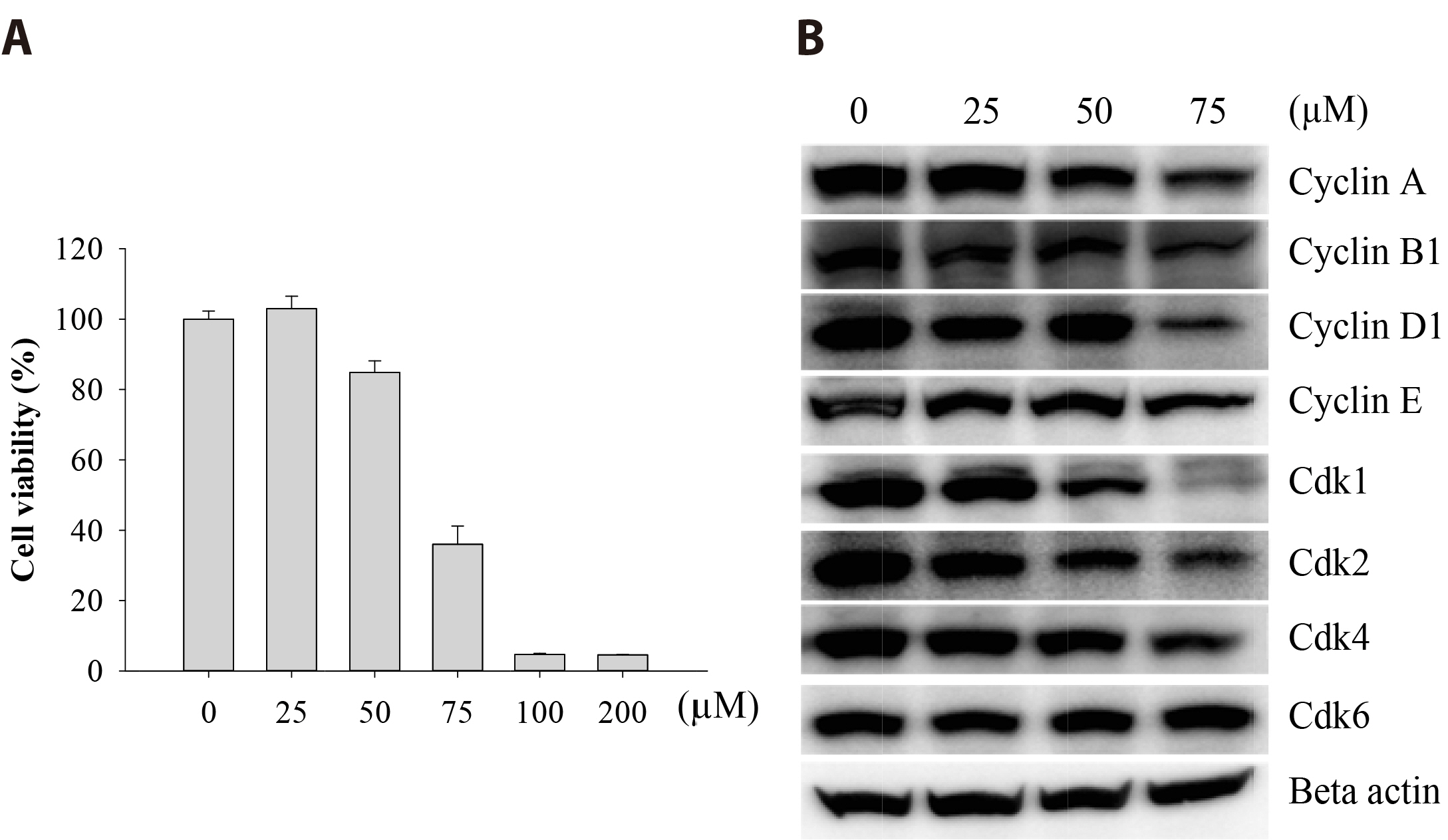
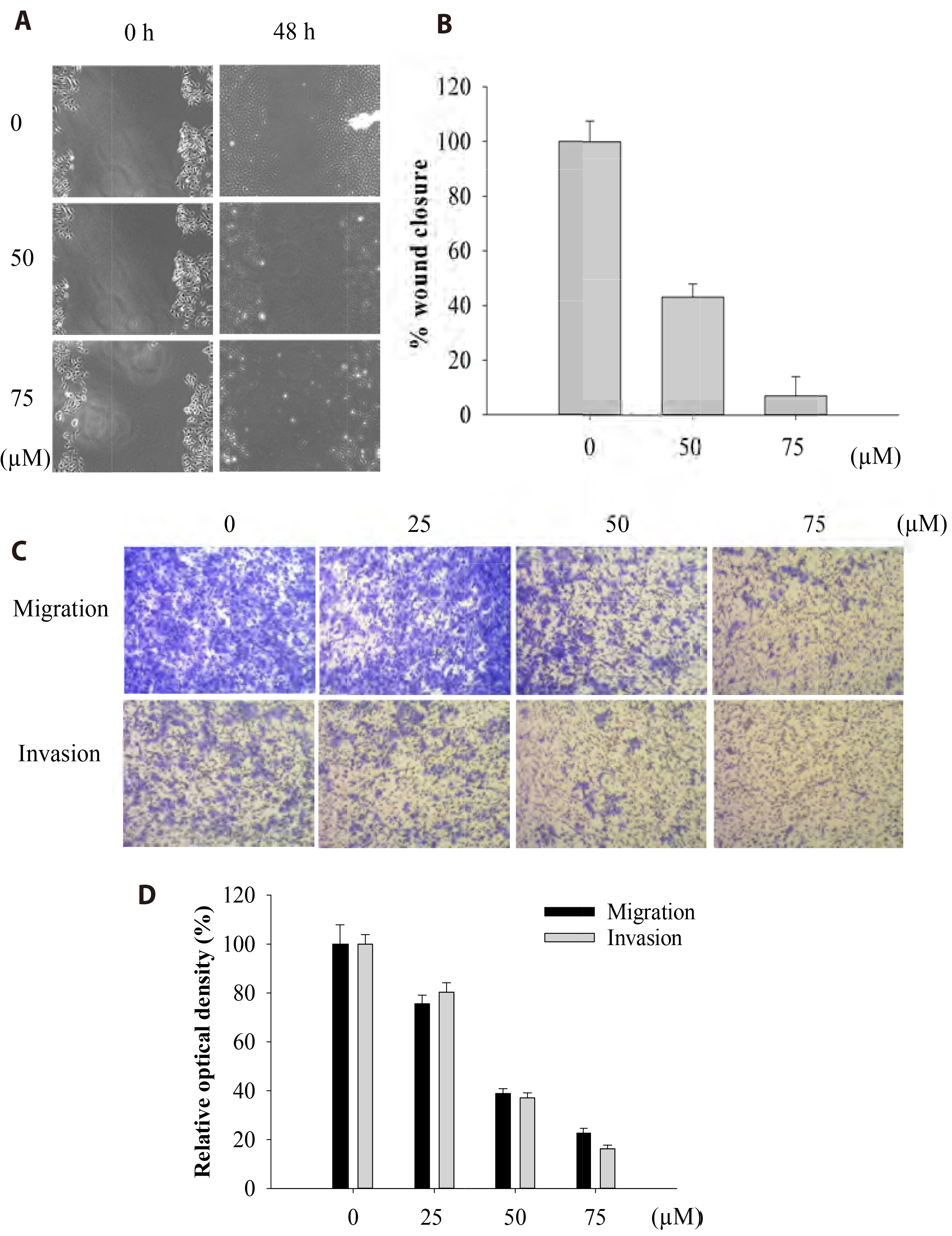
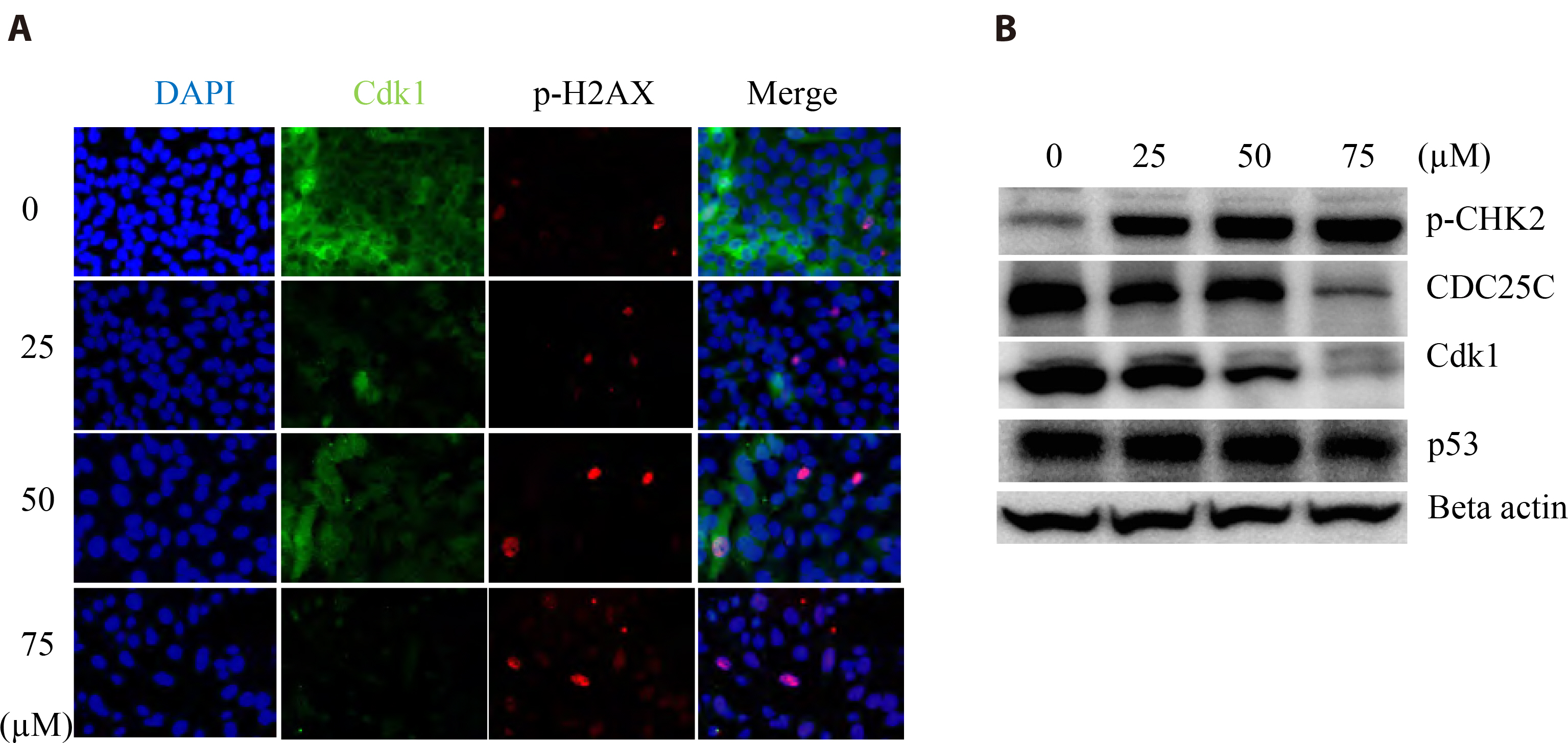
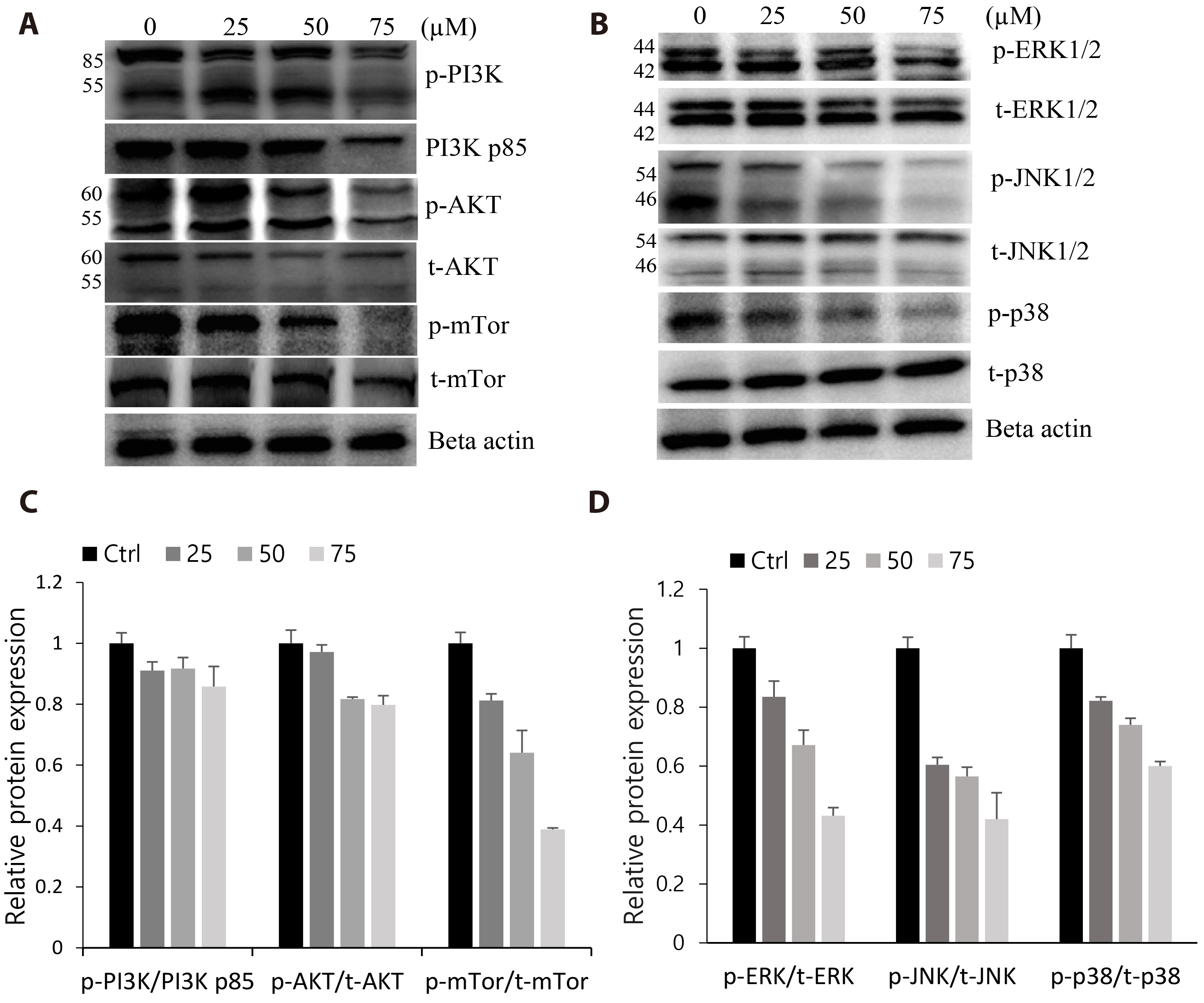
- The antitumoral effects of valdecoxib (Val), an United States Food and Drug Administration-approved anti-inflammatory drug that was withdrawn due to the side effects of increased risk of cardiovascular adverse events, were investigated in hypopharyngeal squamous cell carcinoma cells by performing a cell viability assay, transwell assay, immunofluorescence imaging, and Western blotting. Val markedly inhibited cell viability with an IC50 of 67.3 μM after 48 h of treatment, and also downregulated cell cycle proteins such as Cdks and their regulatory cyclin units. Cell migration and invasion were severely suppressed by inhibiting integrin α4/FAK expression. In addition, Val activated the cell cycle checkpoint CHK2 in response to excessive DNA damage, which led to the activation of caspase-3/9 and induced caspase-dependent apoptosis. Furthermore, the signaling cascades of the PI3K/AKT/ mTOR and mitogen-activated protein kinase pathways were significantly inhibited by Val treatment. Taken together, our results indicate that Val can be used for the treatment of hypopharyngeal squamous cell carcinoma.
Keyword
Figure
Reference
-
1. Lu W, Feng L, Li P, Wang Y, Du Y, Chen X, Wu S, Zhao G, Lou W. 2016; Effects of HPV-16 infection on hypopharyngeal squamous cell carcinoma and FaDu cells. Oncol Rep. 35:99–106. DOI: 10.3892/or.2015.4340. PMID: 26497405. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84954063579&origin=inward.
Article2. Gupta T, Chopra S, Agarwal JP, Laskar SG, D'cruz AK, Shrivastava SK, Dinshaw KA. 2009; Squamous cell carcinoma of the hypopharynx: single-institution outcome analysis of a large cohort of patients treated with primary non-surgical approaches. Acta Oncol. 48:541–548. DOI: 10.1080/02841860802488839. PMID: 18979267. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=65549124151&origin=inward.
Article3. Chang MF, Wang HM, Kang CJ, Huang SF, Lin CY, Fang KH, Chen EY, Chen IH, Liao CT, Chang JT. 2010; Treatment results for hypopharyngeal cancer by different treatment strategies and its secondary primary--an experience in Taiwan. Radiat Oncol. 5:91. DOI: 10.1186/1748-717X-5-91. PMID: 20925962. PMCID: PMC2958972. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77957360232&origin=inward.4. Liu DB, Hu GY, Long GX, Qiu H, Mei Q, Hu GQ. 2012; Celecoxib induces apoptosis and cell-cycle arrest in nasopharyngeal carcinoma cell lines via inhibition of STAT3 phosphorylation. Acta Pharmacol Sin. 33:682–690. Erratum in: Acta Pharmacol Sin. 2013;34:581. DOI: 10.1038/aps.2012.18. PMID: 22504904. PMCID: PMC4010355. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84860785567&origin=inward.
Article5. Schellhorn M, Haustein M, Frank M, Linnebacher M, Hinz B. 2015; Celecoxib increases lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Oncotarget. 6:39342–39356. DOI: 10.18632/oncotarget.5745. PMID: 26513172. PMCID: PMC4770776. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84948808347&origin=inward.
Article6. Li J, Hao Q, Cao W, Vadgama JV, Wu Y. 2018; Celecoxib in breast cancer prevention and therapy. Cancer Manag Res. 10:4653–4667. DOI: 10.2147/CMAR.S178567. PMID: 30464589. PMCID: PMC6208493. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85057735019&origin=inward.
Article7. Inan Genç A, Gok S, Banerjee S, Severcan F. 2017; Valdecoxib recovers the lipid composition, order and dynamics in colon cancer cell lines independent of COX-2 expression: an ATR-FTIR spectroscopy study. Appl Spectrosc. 71:105–117. DOI: 10.1177/0003702816654164. PMID: 27354402. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85008474391&origin=inward.
Article8. Diril MK, Ratnacaram CK, Padmakumar VC, Du T, Wasser M, Coppola V, Tessarollo L, Kaldis P. 2012; Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A. 109:3826–3831. DOI: 10.1073/pnas.1115201109. PMID: 22355113. PMCID: PMC3309725. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84857956418&origin=inward.
Article9. Lindqvist A. 2010; Cyclin B-Cdk1 activates its own pump to get into the nucleus. J Cell Biol. 189:197–199. DOI: 10.1083/jcb.201003032. PMID: 20404105. PMCID: PMC2856906. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77951175503&origin=inward.
Article10. Qian F, Vaux DL, Weissman IL. 1994; Expression of the integrin alpha 4 beta 1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell. 77:335–347. DOI: 10.1016/0092-8674(94)90149-X. PMID: 8181055. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028175008&origin=inward.
Article11. Wu L, Bernard-Trifilo JA, Lim Y, Lim ST, Mitra SK, Uryu S, Chen M, Pallen CJ, Cheung NK, Mikolon D, Mielgo A, Stupack DG, Schlaepfer DD. 2008; Distinct FAK-Src activation events promote alpha5beta1 and alpha4beta1 integrin-stimulated neuroblastoma cell motility. Oncogene. 27:1439–1448. DOI: 10.1038/sj.onc.1210770. PMID: 17828307. PMCID: PMC2593630. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39849104758&origin=inward.
Article12. Young SA, McCabe KE, Bartakova A, Delaney J, Pizzo DP, Newbury RO, Varner JA, Schlaepfer DD, Stupack DG. 2015; Integrin α4 enhances metastasis and may be associated with poor prognosis in MYCN-low neuroblastoma. PLoS One. 10:e0120815. DOI: 10.1371/journal.pone.0120815. PMID: 25973900. PMCID: PMC4431816. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929346852&origin=inward.
Article13. Scalici JM, Harrer C, Allen A, Jazaeri A, Atkins KA, McLachlan KR, Slack-Davis JK. 2014; Inhibition of α4β1 integrin increases ovarian cancer response to carboplatin. Gynecol Oncol. 132:455–461. DOI: 10.1016/j.ygyno.2013.12.031. PMID: 24378876. PMCID: PMC3939448. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84894078697&origin=inward.
Article14. Pulkka OP, Mpindi JP, Tynninen O, Nilsson B, Kallioniemi O, Sihto H, Joensuu H. 2018; Clinical relevance of integrin alpha 4 in gastrointestinal stromal tumours. J Cell Mol Med. 22:2220–2230. DOI: 10.1111/jcmm.13502. PMID: 29377440. PMCID: PMC5867167. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041201597&origin=inward.
Article15. Rieke DT, Klinghammer K, Keilholz U. 2016; Targeted therapy of head and neck cancer. Oncol Res Treat. 39:780–786. DOI: 10.1159/000452432. PMID: 27889751. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84996843220&origin=inward.
Article16. Massacesi C, Di Tomaso E, Urban P, Germa C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R, Hirawat S. 2016; PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther. 9:203–210. DOI: 10.2147/OTT.S89967. PMID: 26793003. PMCID: PMC4708174. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84954357527&origin=inward.
Article17. Renshaw J, Taylor KR, Bishop R, Valenti M, De Haven Brandon A, Gowan S, Eccles SA, Ruddle RR, Johnson LD, Raynaud FI, Selfe JL, Thway K, Pietsch T, Pearson AD, Shipley J. 2013; Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin Cancer Res. 19:5940–5951. DOI: 10.1158/1078-0432.CCR-13-0850. PMID: 23918606. PMCID: PMC3818134. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84887065790&origin=inward.
Article18. Peng X, Liu Y, Zhu S, Peng X, Li H, Jiao W, Lin P, Zhang Z, Qiu Y, Jin M, Wang R, Kong D. 2019; Co-targeting PI3K/Akt and MAPK/ERK pathways leads to an enhanced antitumor effect on human hypopharyngeal squamous cell carcinoma. J Cancer Res Clin Oncol. 145:2921–2936. DOI: 10.1007/s00432-019-03047-2. PMID: 31620898. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85073977066&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antitumor effects of octyl gallate on hypopharyngeal carcinoma cells
- Endoscopic Resection of Hypopharyngeal Squamous Cell Carcinoma
- Is it Necessary to Dissect Level I in Laryngeal and Hypopharyngeal Squamous Cell Carcinoma?
- Apoptosis induced by water extracts of Nypa fruticans wurmb via a mitochondria-dependent pathway in human FaDu hypopharyngeal squamous carcinoma cells
- A Case of Adenoid Squamous Cell Carcinoma