J Liver Cancer.
2022 Sep;22(2):125-135. 10.17998/jlc.2022.05.24.
Effect of direct-acting antivirals for hepatitis C virus-related hepatocellular carcinoma recurrence and death after curative treatment
- Affiliations
-
- 1Department of Gastroenterology, Ajou University School of Medicine, Suwon, Korea
- 2Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea
- 3Office of Biostatistics, Medical Research Collaborating Center, Ajou Research Institute for Innovative Medicine, Ajou University Medical Center, Suwon, Korea
- KMID: 2534239
- DOI: http://doi.org/10.17998/jlc.2022.05.24
Abstract
- Background
/Aim: There has been a long-standing debate about the association of directacting antiviral (DAA) therapy and hepatocellular carcinoma (HCC) recurrence. This study aimed to investigate the association between DAA therapy and HCC recurrence after curative therapy.
Methods
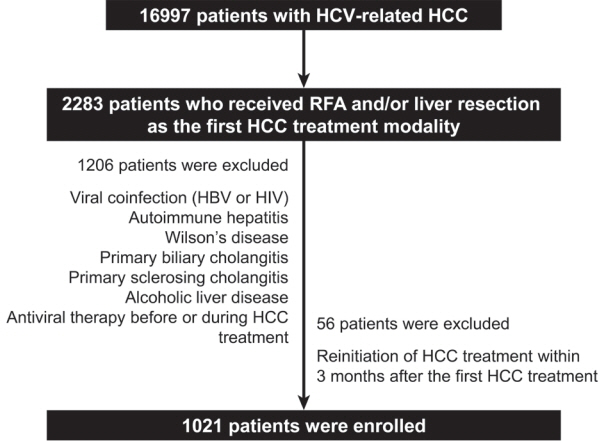
We retrospectively enrolled 1,021 patients with HCV-related (hepatitis C virus) HCC who underwent radiofrequency ablation (RFA), liver resection, or both as the first treatment modality from January 2007 to December 2016 and without a history of HCV therapy before HCC treatment from a nationwide database. The effect of HCV treatment on HCC recurrence and all-cause mortality was also investigated.
Results
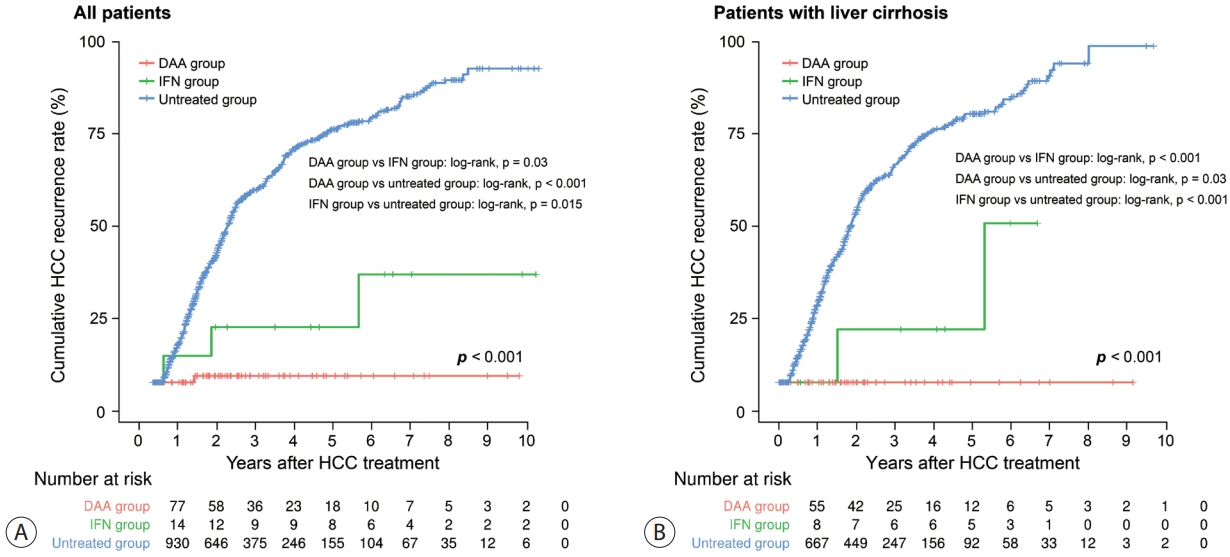
Among the 1,021 patients, 77 (7.5%) were treated with DAA, 14 (1.4%) were treated with interferon-based therapy, and 930 (91.1%) did not receive HCV therapy. DAA therapy was an independent prognostic factor for lower HCC recurrence rate (hazard ratio [HR], 0.04; 95% confidence interval [CI], 0.006-0.289; P=0.001 for landmarks at 6 months after HCC treatment and HR, 0.05; 95% CI, 0.007-0.354; P=0.003 for landmarks at 1 year). Furthermore, DAA therapy was associated with lower all-cause mortality (HR, 0.049; 95% CI, 0.007-0.349; P=0.003 for landmarks at 6 months and HR, 0.063; 95% CI, 0.009-0.451; P=0.006 for landmarks at 1 year).
Conclusions
DAA therapy after curative HCC treatment can decrease HCC recurrence and all-cause mortality compared to interferon-based therapy or no antiviral therapy. Therefore, clinicians should consider administering DAA therapy after curative HCC treatment in patients with HCV-related HCC.
Figure
Reference
-
1. Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000; 343:1666–1672.2. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001; 358:958–965.3. Lawitz E, Makara M, Akarca US, Thuluvath PJ, Preotescu LL, Varunok P, et al. Efficacy and safety of ombitasvir, paritaprevir, and ritonavir in an open-label study of patients with genotype 1b chronic hepatitis C virus infection with and without cirrhosis. Gastroenterology. 2015; 149:971–980. e1.4. Cho BW, Kim SB, Song IH, Lee SH, Kim HS, Lee TH, et al. Efficacy and safety of daclatasvir plus asunaprevir for Korean patients with HCV genotype Ib infection: a retrospective multi-institutional study. Clin Mol Hepatol. 2017; 23:51–56.5. World Health Organization (WHO). Global hepatitis report, 2017 [Internet]. Geneva: WHO; [cited 2019 Jan 1]. Available from: https://www.who.int/publications/i/item/9789241565455.6. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017; 2:161–176.7. Hanouneh IA, Alkhouri N, Singal AG. Hepatocellular carcinoma surveillance in the 21st century: saving lives or causing harm? Clin Mol Hepatol. 2019; 25:264–269.8. Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016; 65:719–726.9. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016; 65:727–733.10. Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther. 2017; 46:688–695.11. Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017; 67:933–939.12. Minami T, Tateishi R, Nakagomi R, Fujiwara N, Sato M, Enooku K, et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol. 2016; 65:1272–1273.13. Nagaoki Y, Imamura M, Nishida Y, Daijo K, Teraoka Y, Honda F, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019; 91:650–658.14. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017; 32:718–728.15. Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013; 31:2963–2969.16. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999; 18:695–706.17. ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol. 2016; 65:734–740.18. Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology. 2019; 156:1683–1692. e1.19. Lin WC, Lin YS, Chang CW, Chang CW, Wang TE, Wang HY, et al. Impact of direct-acting antiviral therapy for hepatitis C-related hepatocellular carcinoma. PLoS One. 2020; 15:e0233212.20. Shen YC, Hsu C, Chen LT, Cheng CC, Hu FC, Cheng AL. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J Hepatol. 2010; 52:889–894.21. Hsu CS, Chao YC, Lin HH, Chen DS, Kao JH. Systematic review: impact of interferon-based therapy on HCV-related hepatocellular carcinoma. Sci Rep. 2015; 5:9954.22. Kusano H, Akiba J, Ogasawara S, Sanada S, Yasumoto M, Nakayama M, et al. Pegylated interferon-α2a inhibits proliferation of human liver cancer cells in vitro and in vivo. PLoS One. 2013; 8:e83195.23. Kuo YH, Wang JH, Chang KC, Hung CH, Lu SN, Hu TH, et al. The influence of direct-acting antivirals in hepatitis C virus related hepatocellular carcinoma after curative treatment. Invest New Drugs. 2020; 38:202–210.24. Teng W, Jeng WJ, Yang HI, Chen WT, Hsieh YC, Huang CH, et al. Interferon is superior to direct acting antiviral therapy in tertiary prevention of early recurrence of hepatocellular carcinoma. Cancers (Basel). 2019; 12:23.25. Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful interferon-free therapy of chronic hepatitis C Virus infection normalizes natural killer cell function. Gastroenterology. 2015; 149:190–200. e2.26. Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017; 67:1204–1212.27. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.28. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.29. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994; 81:515–526.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Potential Therapies for Chronic Hepatitis B
- Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy
- Updated Treatment of Chronic Hepatitis C
- Management of viral hepatitis in patients with hepatocellular carcinoma
- Two Cases of Early Recurred Hepatocellular Carcinoma after Surgical Resection Which Showed Different Outcomes