Cancer Res Treat.
2022 Oct;54(4):1219-1229. 10.4143/crt.2021.1010.
Identification of Patients with Recurrent Epithelial Ovarian Cancer Who Will Benefit from More Than Three Lines of Chemotherapy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Hospital, Korea
- 2Department of Obstetrics and Gynecology, Dongguk University College of Medicine, Goyang, Korea
- 3Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2534201
- DOI: http://doi.org/10.4143/crt.2021.1010
Abstract
- Purpose
This study aimed to identify patients who would benefit from third and subsequent lines of chemotherapy in recurrent epithelial ovarian cancer (EOC).
Materials and Methods
Recurrent EOC patients who received third, fourth, or fifth-line palliative chemotherapy were retrospectively analyzed. Patients’ survival outcomes were assessed according to chemotherapy lines. Based on the best objective response, patients were divided into good-response (stable disease or better) and poor response (progressive disease or those who died before response assessment) groups. Survival outcomes were compared between the two groups, and factors associated with chemotherapy responses were investigated.
Results
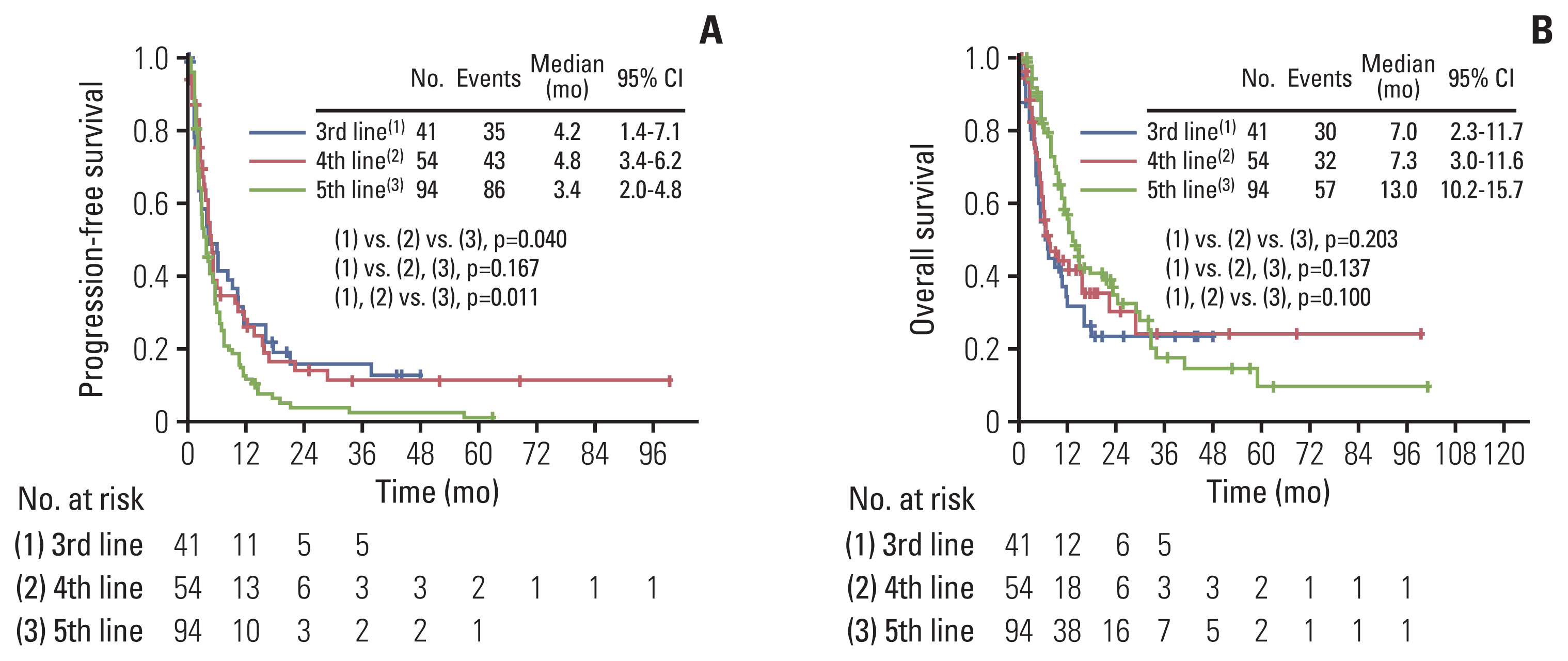
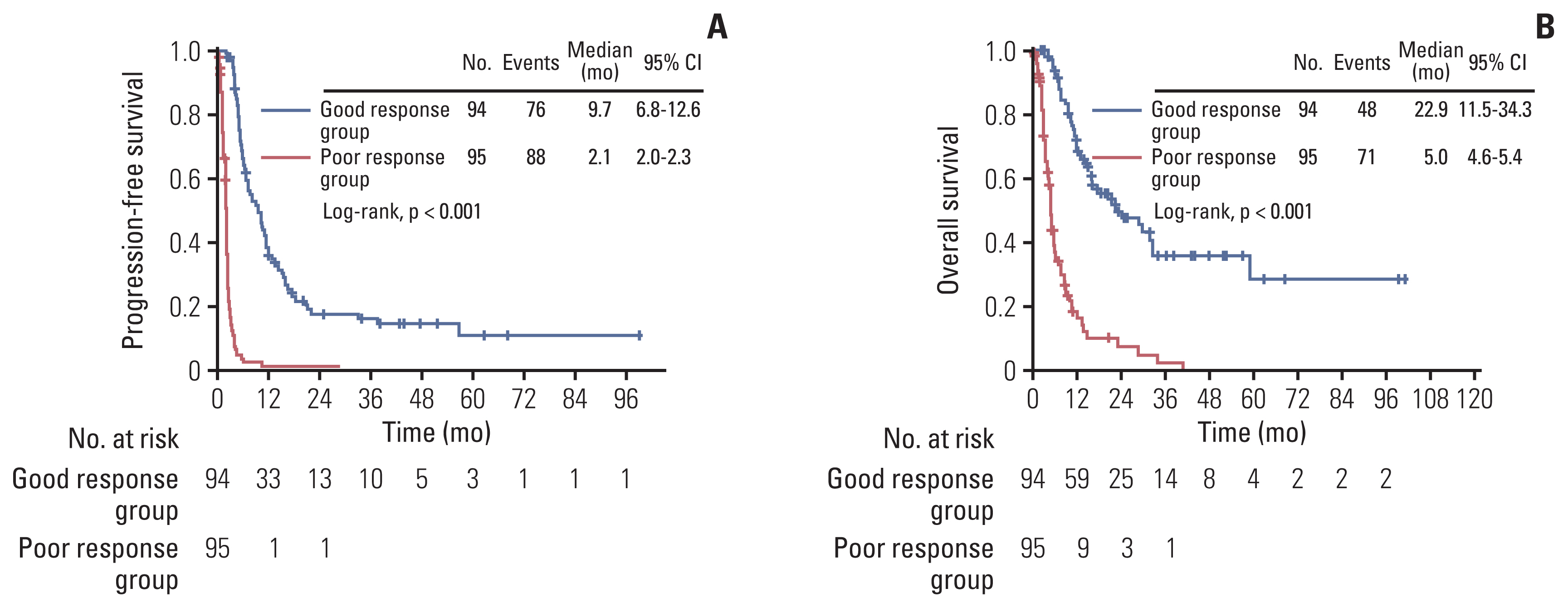
A total of 189 patients were evaluated. Ninety-four and 95 patients were identified as good and poor response group respectively, during the study period of 2008 to 2021. The poor response group showed significantly worse progression-free survival (median, 2.1 months vs. 9.7 months; p < 0.001) and overall survival (median, 5.0 months vs. 22.9 months; p < 0.001) compared with the good response group. In multivariate analysis adjusting for clinicopathologic factors, short treatment-free interval (TFI) (hazard ratio [HR], 5.557; 95% confidence interval [CI], 2.403 to 12.850), platinum-resistant EOC (HR, 2.367; 95% CI, 1.017 to 5.510), and non-serous/endometrioid histologic type (HR, 5.045; 95% CI, 1.152 to 22.088) were identified as independent risk factors for poor response. There was no difference in serious adverse events between good and poor response groups (p=0.167).
Conclusion
Third and subsequent lines of chemotherapy could be carefully considered for palliative purposes in recurrent EOC patients with serous or endometrioid histology, initial platinum sensitivity, and long TFIs from the previous chemotherapy regimen.
Keyword
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article3. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–917.
Article4. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–200.
Article5. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014; 384:1376–88.
Article6. Herzog TJ. Recurrent ovarian cancer: how important is it to treat to disease progression? Clin Cancer Res. 2004; 10:7439–49.7. Browner I, Carducci MA. Palliative chemotherapy: historical perspective, applications, and controversies. Semin Oncol. 2005; 32:145–55.
Article8. Jang TK, Kim DY, Lee SW, Park JY, Suh DS, Kim JH, et al. Trends in treatment during the last stages of life in end-stage gynecologic cancer patients who received active palliative chemotherapy: a comparative analysis of 10-year data in a single institution. BMC Palliat Care. 2018; 17:99.
Article9. Ventafridda V. According to the 2002 WHO definition of palliative care. Palliat Med. 2006; 20:159.
Article10. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004; 22:315–21.
Article11. Murillo JR Jr, Koeller J. Chemotherapy given near the end of life by community oncologists for advanced non-small cell lung cancer. Oncologist. 2006; 11:1095–9.
Article12. Watanabe-Galloway S, Zhang W, Watkins K, Islam KM, Nayar P, Boilesen E, et al. Quality of end-of-life care among rural Medicare beneficiaries with colorectal cancer. J Rural Health. 2014; 30:397–405.
Article13. Urban RR, He H, Alfonso R, Hardesty MM, Goff BA. The end of life costs for Medicare patients with advanced ovarian cancer. Gynecol Oncol. 2018; 148:336–41.
Article14. Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001; 134:1096–105.
Article15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article16. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Bethesda, MD: National Institutes of Health;2009.17. Giorgi F, Bascioni R, Brugni M, Safi M, Berardi R, Giustini L, et al. Chemotherapy use at the end of life: an analysis of the decision making process. J Clin Oncol. 2004; 22:6081.
Article18. Dans M, Kutner JS, Agarwal R, Baker JN, Bauman JR, Beck AC, et al. NCCN Guidelines(R) insights: palliative care, version 2.2021. J Natl Compr Canc Netw. 2021; 19:780–8.19. Hui D, Kim SH, Roquemore J, Dev R, Chisholm G, Bruera E. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014; 120:1743–9.
Article20. Harrington SE, Smith TJ. The role of chemotherapy at the end of life: “when is enough, enough?”. JAMA. 2008; 299:2667–78.21. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–32.
Article22. Massarelli E, Andre F, Liu DD, Lee JJ, Wolf M, Fandi A, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003; 39:55–61.
Article23. Griffiths RW, Zee YK, Evans S, Mitchell CL, Kumaran GC, Welch RS, et al. Outcomes after multiple lines of chemotherapy for platinum-resistant epithelial cancers of the ovary, peritoneum, and fallopian tube. Int J Gynecol Cancer. 2011; 21:58–65.
Article24. Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer. 1999; 7:128–33.
Article25. Chou WC, Kao CY, Wang PN, Chang H, Wang HM, Chang PH, et al. The application of the Palliative Prognostic Index, charlson comorbidity index, and Glasgow Prognostic Score in predicting the life expectancy of patients with hematologic malignancies under palliative care. BMC Palliat Care. 2015; 14:18.
Article26. Prigerson HG, Bao Y, Shah MA, Paulk ME, LeBlanc TW, Schneider BJ, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015; 1:778–84.
Article27. Rose PG, Java JJ, Salani R, Geller MA, Secord AA, Tewari KS, et al. Nomogram for predicting individual survival after recurrence of advanced-stage, high-grade ovarian carcinoma. Obstet Gynecol. 2019; 133:245–54.
Article28. Tang ST, Wu SC, Hung YN, Chen JS, Huang EW, Liu TW. Determinants of aggressive end-of-life care for Taiwanese cancer decedents, 2001 to 2006. J Clin Oncol. 2009; 27:4613–8.
Article29. Hui D, Didwaniya N, Vidal M, Shin SH, Chisholm G, Roquemore J, et al. Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer. 2014; 120:1572–8.
Article30. He Y, Li T, Liu J, Ou Q, Zhou J. Early onset neutropenia: a useful predictor of chemosensitivity and favorable prognosis in patients with serous ovarian cancer. BMC Cancer. 2020; 20:116.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for ovarian cancer
- Pitfall in Chemotherapy for Ovarian Cancer
- Advanced ovarian cancer: what should be the standard of care?
- The role of topotecan as second-line chemotherapy in patients with recurrent epithelial ovarian cancer
- Prognostic factors of secondary cytoreductive surgery for patients with recurrent epithelial ovarian cancer