Cancer Res Treat.
2022 Oct;54(4):1175-1190. 10.4143/crt.2021.1133.
Histopathologic and Molecular Biomarkers of PD-1/PD-L1 Inhibitor Treatment Response among Patients with Microsatellite Instability‒High Colon Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Asan Center for Cancer Genome Discovery, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Medical Science, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2534197
- DOI: http://doi.org/10.4143/crt.2021.1133
Abstract
- Purpose
Recent clinical trials have reported response rates < 50% among patients treated with programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors for microsatellite instability‒high (MSI-H) colorectal cancer (CRC), and factors predicting treatment response have not been fully identified. This study aimed to identify potential biomarkers of PD-1/PD-L1 inhibitor treatment response among patients with MSI-H CRC.
Materials and Methods
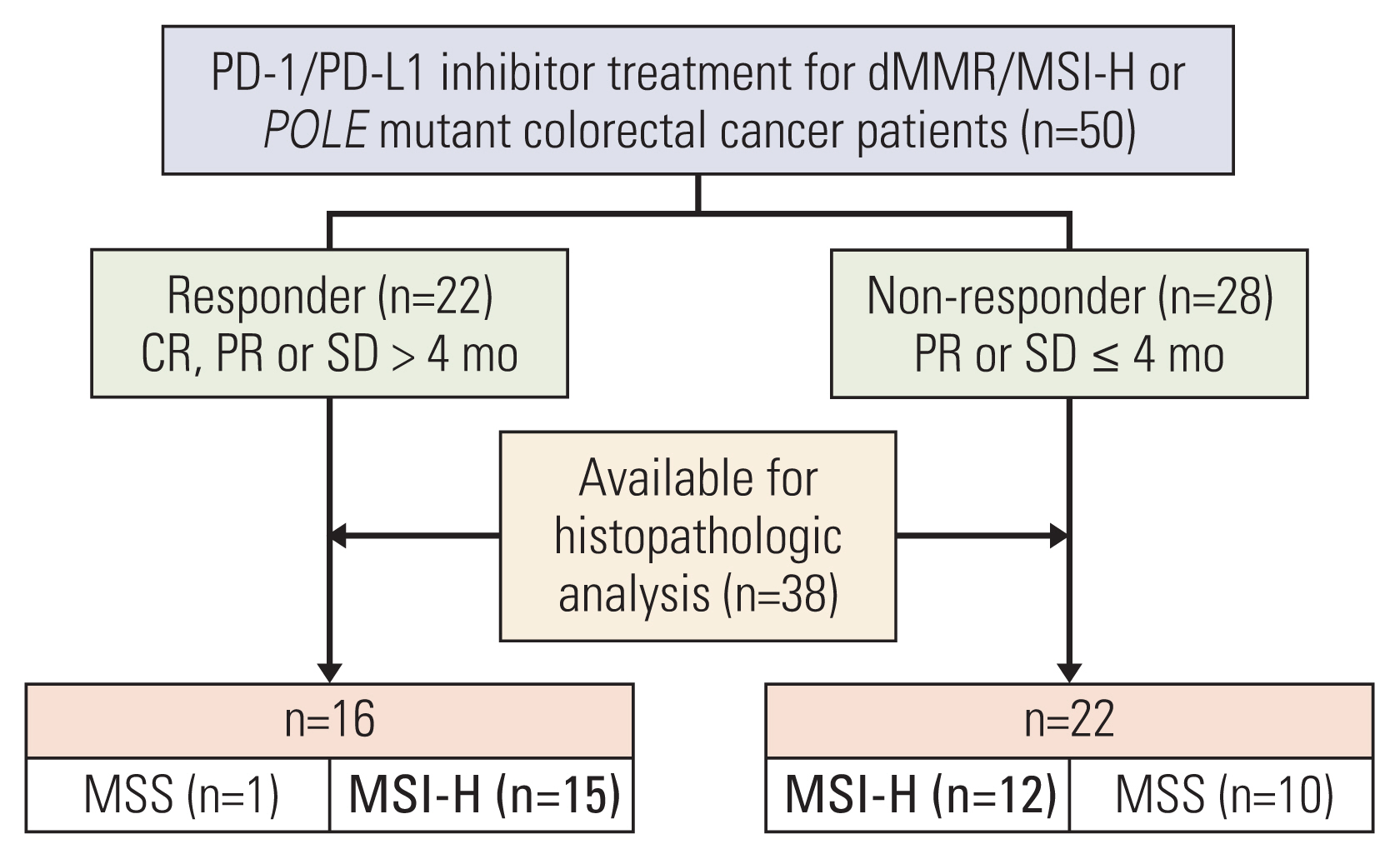
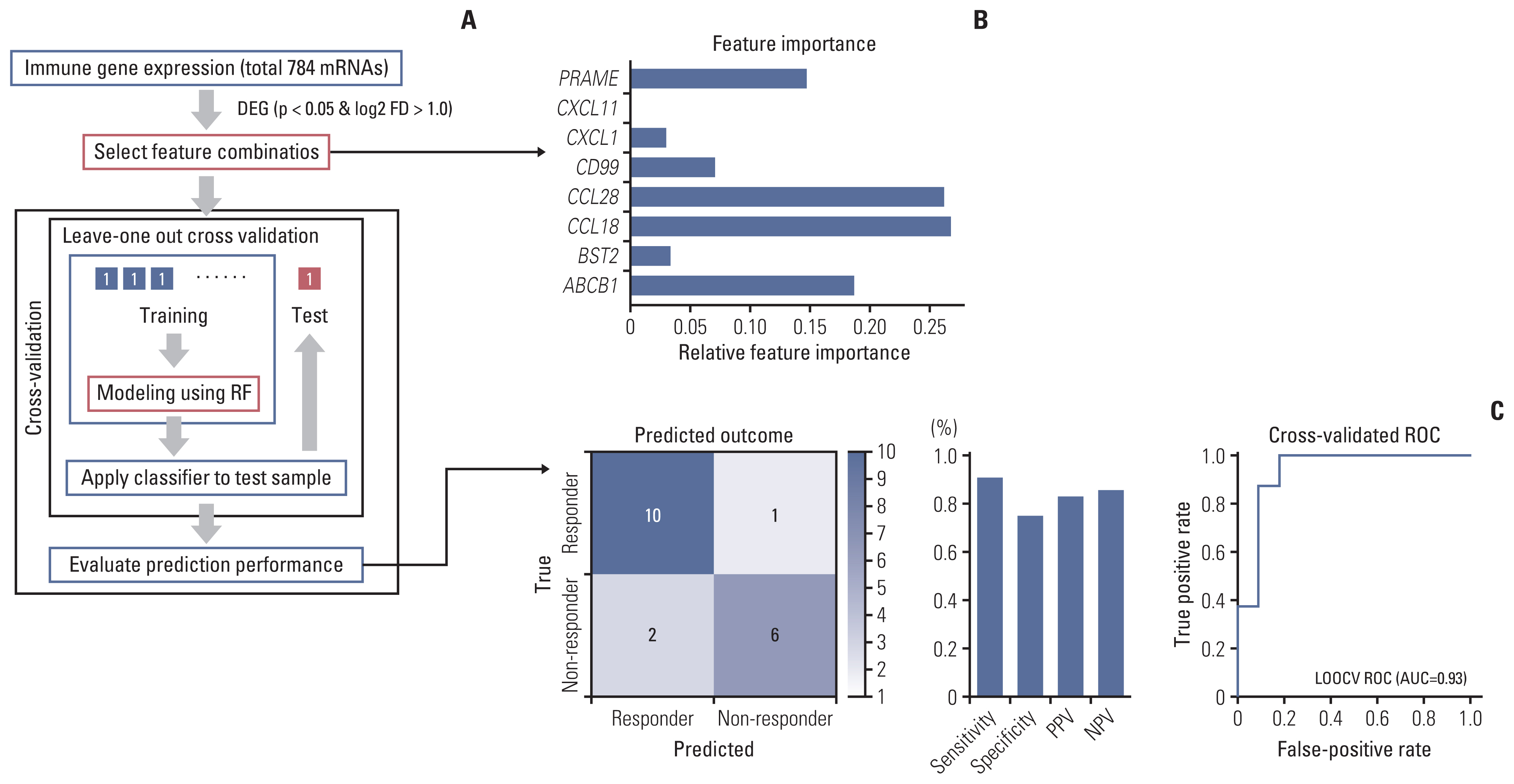
MSI-H CRC patients enrolled in three clinical trials of PD-1/PD-L1 blockade at Asan Medical Center (Seoul, Republic of Korea) were screened and classified into two groups according to treatment response. Their histopathologic features and expression of 730 immune-related genes from the NanoString platform were evaluated, and a machine learning–based classification model was built to predict treatment response among MSI-H CRCs patients.
Results
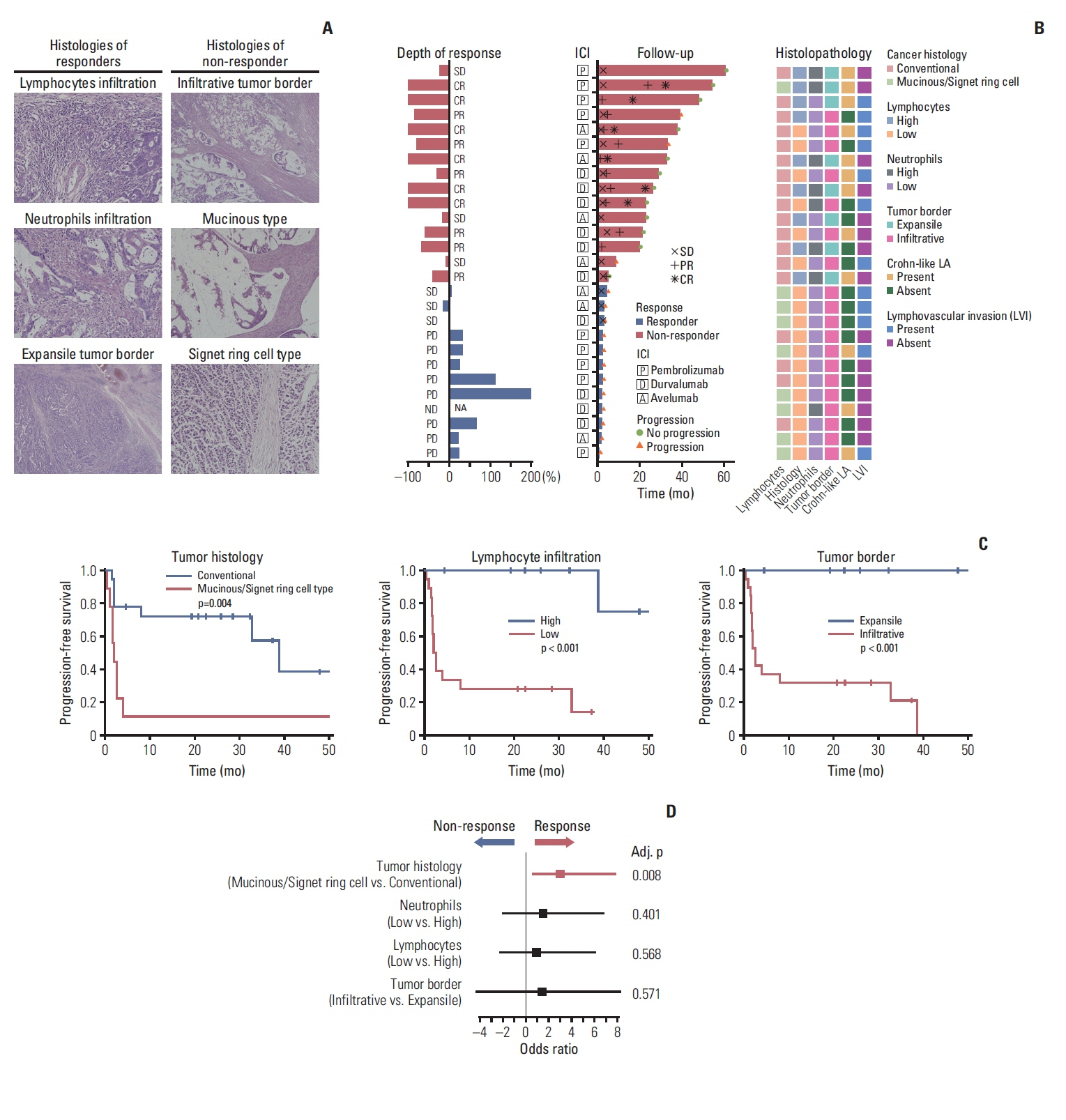
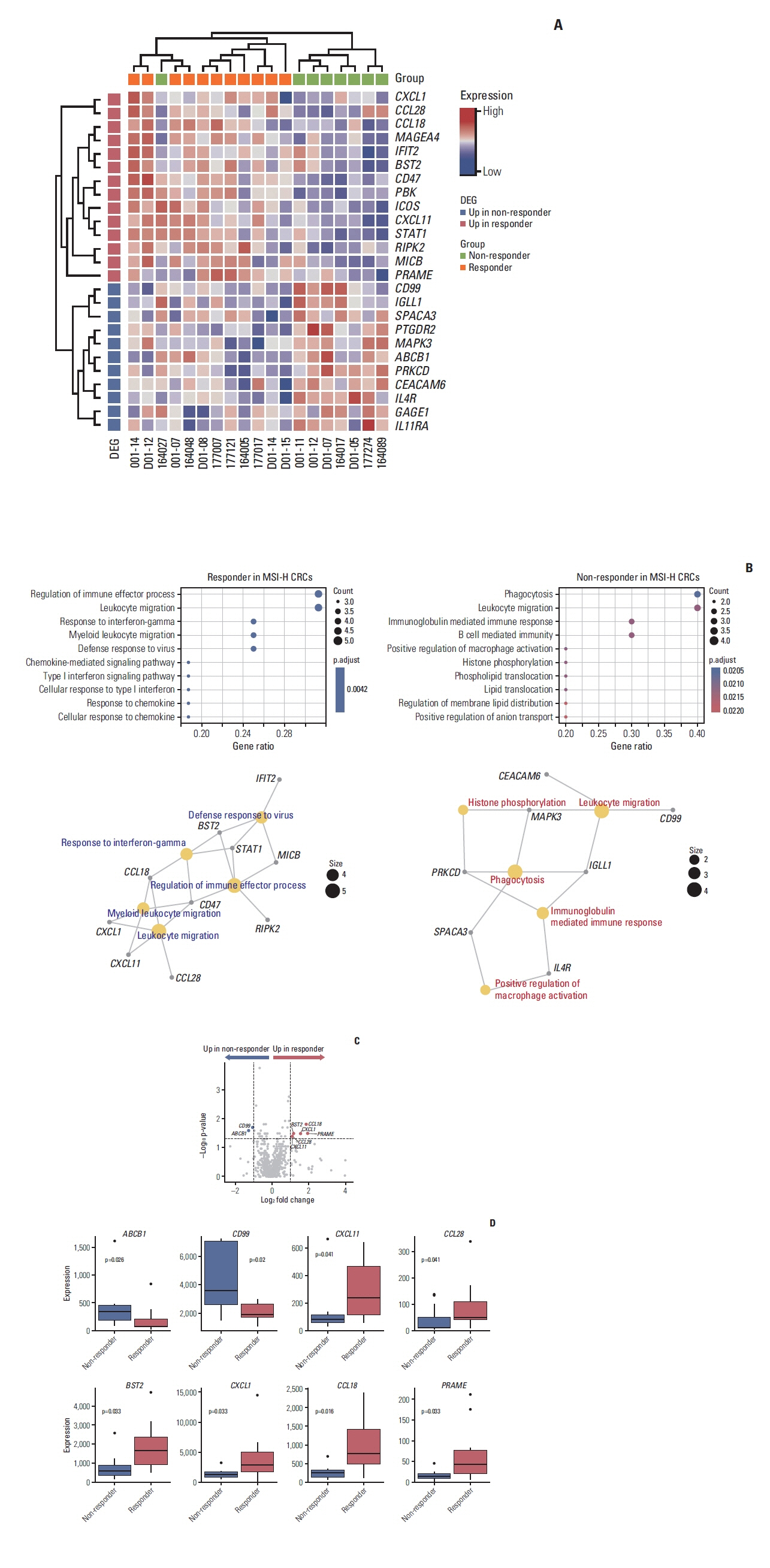
A total of 27 patients (15 responders, 12 non-responders) were included. A high degree of lymphocytic/neutrophilic infiltration and an expansile tumor border were associated with treatment response and prolonged progression-free survival (PFS), while mucinous/signet-ring cell carcinoma was associated with a lack of treatment response and short PFS. Gene expression profiles revealed that the interferon-γ response pathway was enriched in the responder group. Of the top eight differentially expressed immune-related genes, PRAME had the highest fold change in the responder group. Higher expression of PRAME was independently associated with better PFS along with histologic subtypes in the multivariate analysis. The classification model using these genes showed good performance for predicting treatment response.
Conclusion
We identified histologic and immune-related gene expression characteristics associated with treatment response in MSI-H CRC, which may contribute to optimal patient stratification.
Keyword
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Atreya CE, Yaeger R, Chu E. Systemic therapy for metastatic colorectal cancer: from current standards to future molecular targeted approaches. Am Soc Clin Oncol Educ Book. 2017; 37:246–56.
Article3. Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014; 20:5322–30.4. National Comprehensive Cancer Network. Colon cancer (version 1, 2021) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;c2021. [cited 2021 Jan 21]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf .5. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010; 138:2073–87.
Article6. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.7. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017; 18:1182–91.
Article8. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020; 383:2207–18.
Article9. Kim JH, Kim SY, Baek JY, Cha YJ, Ahn JB, Kim HS, et al. A phase II study of avelumab monotherapy in patients with mismatch repair-deficient/microsatellite instability-high or POLE-mutated metastatic or unresectable colorectal cancer. Cancer Res Treat. 2020; 52:1135–44.10. Durvalumab for MSI-H or POLE mutated metastatic colorectal cancer [Internet]. Bethesda, MD: US National Library of Medicine;2020. [cited 2021 Jan 21]. Available from: https://ClinicalTrials.gov/show/NCT03435107 .11. Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020; 38:11–9.
Article12. Kim JE, Chun SM, Hong YS, Kim KP, Kim SY, Kim J, et al. Mutation burden and I index for detection of microsatellite instability in colorectal cancer by targeted next-generation sequencing. J Mol Diagn. 2019; 21:241–50.
Article13. Cohen R, Buhard O, Cervera P, Hain E, Dumont S, Bardier A, et al. Clinical and molecular characterisation of hereditary and sporadic metastatic colorectal cancers harbouring microsatellite instability/DNA mismatch repair deficiency. Eur J Cancer. 2017; 86:266–74.
Article14. Guyot D’Asnieres De Salins A, Tachon G, Cohen R, Karayan-Tapon L, Junca A, Frouin E, et al. Discordance between immunochemistry of mismatch repair proteins and molecular testing of microsatellite instability in colorectal cancer. ESMO Open. 2021; 6:100120.
Article15. Agilent Technologies. Instructions for use: PD-L1 IHC 22C3 pharmDx. Santa Clara, CA: Agilent Technologies;2018.16. Funkhouser WK Jr, Lubin IM, Monzon FA, Zehnbauer BA, Evans JP, Ogino S, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn. 2012; 14:91–103.
Article17. Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001; 158:527–35.
Article18. Halvarsson B, Anderson H, Domanska K, Lindmark G, Nilbert M. Clinicopathologic factors identify sporadic mismatch repair-defective colon cancers. Am J Clin Pathol. 2008; 129:238–44.
Article19. Jenkins MA, Hayashi S, O’Shea AM, Burgart LJ, Smyrk TC, Shimizu D, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007; 133:48–56.
Article20. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019; 19:133–50.
Article21. Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018; 391:2128–39.22. Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011; 179:37–45.
Article23. Berry RS, Xiong MJ, Greenbaum A, Mortaji P, Nofchissey RA, Schultz F, et al. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS One. 2017; 12:e0188799.
Article24. Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016; 44:698–711.
Article25. Boissiere-Michot F, Lazennec G, Frugier H, Jarlier M, Roca L, Duffour J, et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology. 2014; 3:e29256.
Article26. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017; 127:2930–40.27. Stoll G, Pol J, Soumelis V, Zitvogel L, Kroemer G. Impact of chemotactic factors and receptors on the cancer immune infiltrate: a bioinformatics study revealing homogeneity and heterogeneity among patient cohorts. Oncoimmunology. 2018; 7:e1484980.28. Epping MT, Bernards R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006; 66:10639–42.
Article29. Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019; 30:1096–103.30. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018; 362:eaar3593.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy
- PD-1: A Negative Regulator of Phagocytosis by Tumour-Associated Macrophages in Colon Cancer
- Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors
- Evaluation of circulating PD-1 and PD-L1 as diagnostic biomarkers in dogs with tumors
- Is a Microsatellite Instability Still Useful for Tailored Treatment in Stage II and III Colon Cancer?