Korean J Pain.
2022 Oct;35(4):391-402. 10.3344/kjp.2022.35.4.391.
The role of botulinum toxin type A related axon transport in neuropathic pain induced by chronic constriction injury
- Affiliations
-
- 1Center of Pain Management, Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Zhengzhou University Academy of Medical Sciences, Zhengzhou, China
- 3Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Neuroscience Research Institute, Zhengzhou University Academy of Medical Sciences, Zhengzhou, China
- 5School of Basic Medical Science, Zhengzhou University, Zhengzhou, China
- KMID: 2533979
- DOI: http://doi.org/10.3344/kjp.2022.35.4.391
Abstract
- Background
The mechanism of peripheral axon transport in neuropathic pain is still unclear. Chemokine ligand 13 (CXCL13) and its receptor (C-X-C chemokine receptor type 5, CXCR5) as well as GABA transporter 1 (GAT-1) play an important role in the development of pain. The aim of this study was to explore the axonal transport of CXCL13/CXCR5 and GAT-1 with the aid of the analgesic effect of botulinum toxin type A (BTX-A) in rats.
Methods
Chronic constriction injury (CCI) rat models were established. BTX-A was administered to rats through subcutaneous injection in the hind paw. The pain behaviors in CCI rats were measured by paw withdrawal threshold and paw withdrawal latencies. The levels of CXCL13/CXCR5 and GAT-1 were measured by western blots.
Results
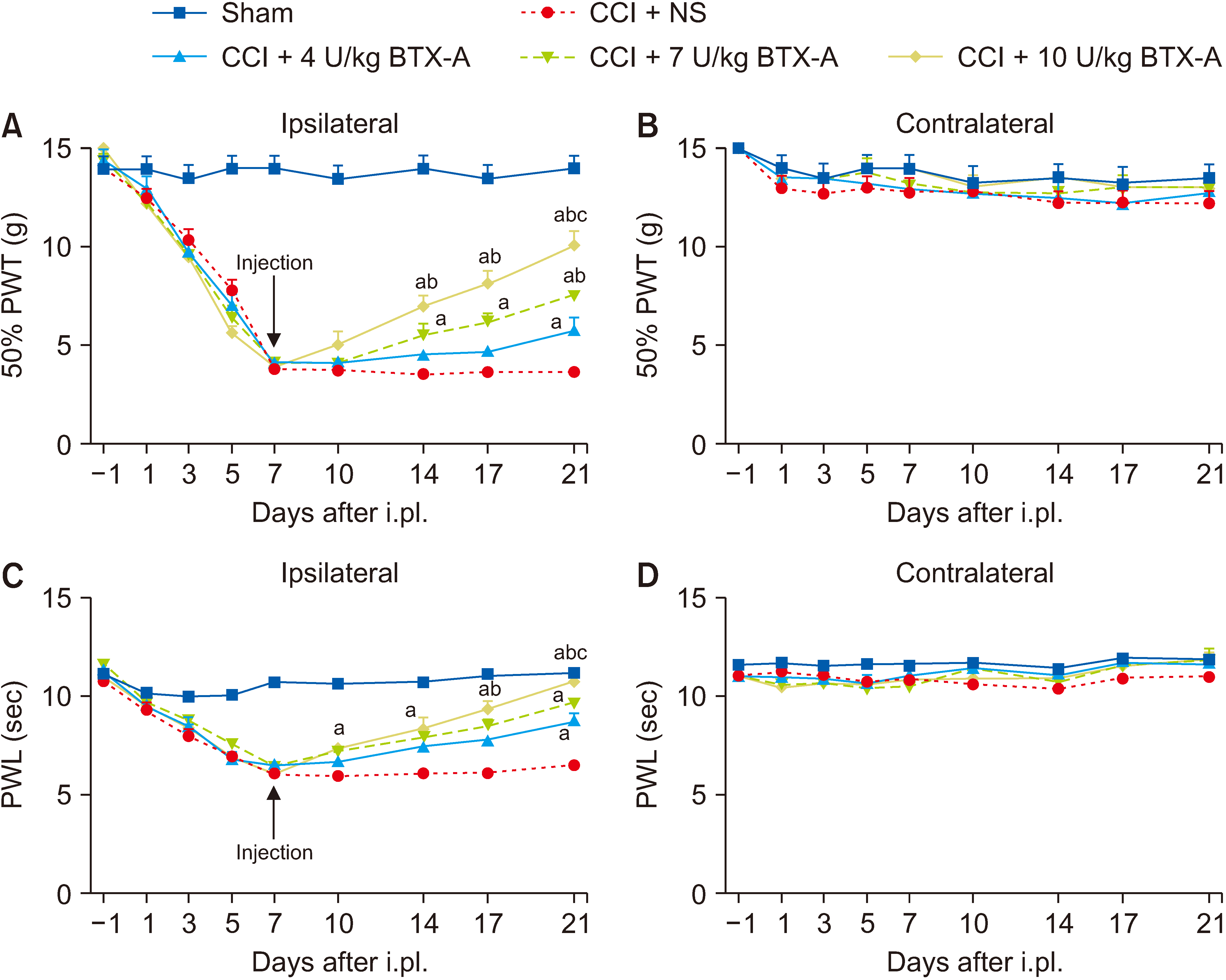
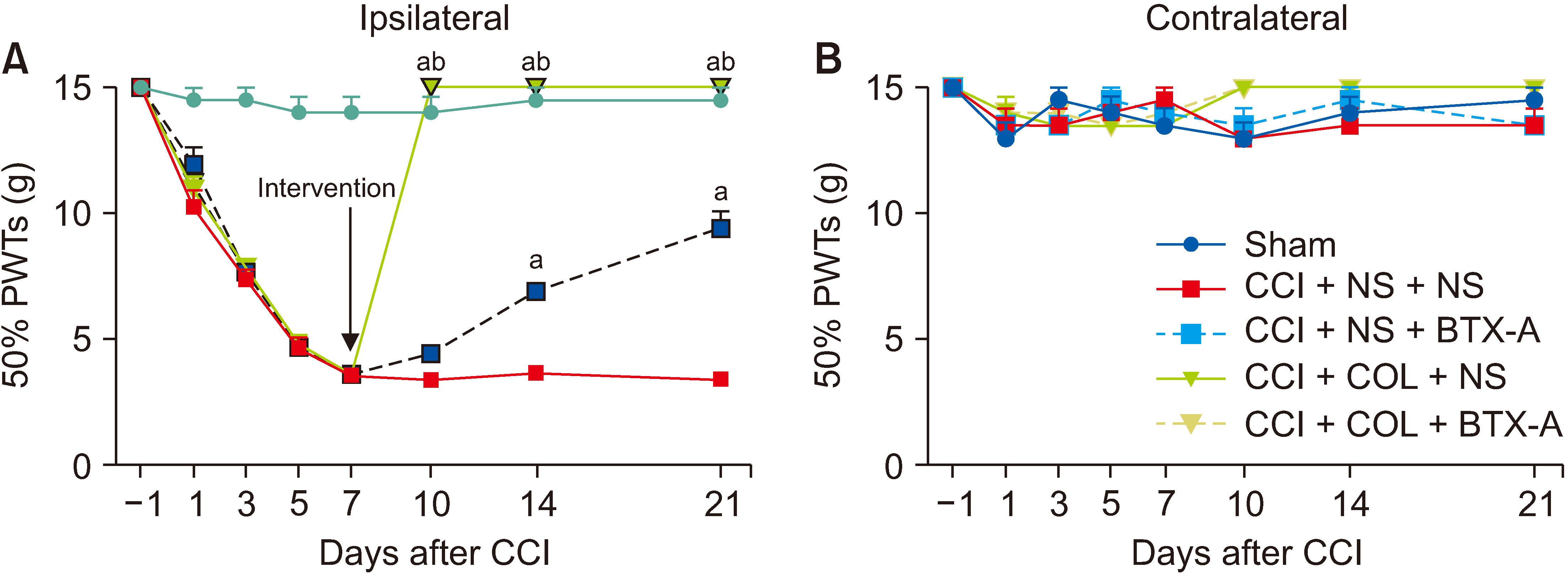
The subcutaneous injection of BTX-A relieved the mechanical allodynia and heat hyperalgesia induced by CCI surgery and reversed the overexpression of CXCL13/CXCR5 and GAT-1 in the spinal cord, dorsal root ganglia (DRG), sciatic nerve, and plantar skin in CCI rats. After 10 mmol/L colchicine blocked the axon transport of sciatic nerve, the inhibitory effect of BTX-A disappeared, and the levels of CXCL13/CXCR5 and GAT-1 in the spinal cord and DRG were reduced in CCI rats.
Conclusions
BTX-A regulated the levels of CXCL13/CXCR5 and GAT-1 in the spine and DRG through axonal transport. Chemokines (such as CXCL13) may be transported from the injury site to the spine or DRG through axonal transport. Axon molecular transport may be a target to enhance pain management in neuropathic pain.
Keyword
Figure
Reference
-
1. Baba M, Takatsuna H, Matsui N, Ohwada S. 2020; Mirogabalin in Japanese patients with renal impairment and pain associated with diabetic peripheral neuropathy or post-herpetic neuralgia: a phase III, open-label, 14-week study. J Pain Res. 13:1811–21. DOI: 10.2147/JPR.S255345. PMID: 32765056. PMCID: PMC7381826. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088415306&origin=inward.2. Rodríguez MJ, Díaz S, Vera-Llonch M, Dukes E, Rejas J. 2007; Cost-effectiveness analysis of pregabalin versus gabapentin in the management of neuropathic pain due to diabetic polyneuropathy or post-herpetic neuralgia. Curr Med Res Opin. 23:2585–96. DOI: 10.1185/030079907X233151. PMID: 17875242. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35648987172&origin=inward.
Article3. Gerken JD, Fritzsche T, Denke C, Schäfer M, Tafelski S. 2020; Retrospective study on ganglionic and nerve block series as therapeutic option for chronic pain patients with refractory neuropathic pain. Pain Res Manag. 2020:6042941. DOI: 10.1155/2020/6042941. PMID: 32774567. PMCID: PMC7399767. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089321936&origin=inward.
Article4. Liu B, Yang Y, Zhang Z, Wang H, Fan B, Sima L. 2020; Clinical study of spinal cord stimulation and pulsed radiofrequency for management of herpes zoster-related pain persisting beyond acute phase in elderly patients. Pain Physician. 23:263–70. PMID: 32517392. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085974146&origin=inward.5. Li Z, Guo Y, Ren X, Rong L, Huang M, Cao J, et al. 2019; HDAC2, but not HDAC1, regulates Kv1.2 expression to mediate neuropathic pain in CCI rats. Neuroscience. 408:339–48. DOI: 10.1016/j.neuroscience.2019.03.033. PMID: 31022463. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065047403&origin=inward.
Article6. Tal M, Eliav E. 1996; Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 64:511–8. DOI: 10.1016/0304-3959(95)00175-1. PMID: 8783316. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029893712&origin=inward.
Article7. Zhang JM, Li H, Munir MA. 2004; Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 109:143–9. DOI: 10.1016/j.pain.2004.01.033. PMID: 15082136. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=1842786067&origin=inward.
Article8. Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, et al. 2012; Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 153:42–55. DOI: 10.1016/j.pain.2011.08.015. PMID: 21993185. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84155162846&origin=inward.
Article9. Li L, Shao J, Wang J, Liu Y, Zhang Y, Zhang M, et al. 2019; MiR-30b-5p attenuates oxaliplatin-induced peripheral neuropathic pain through the voltage-gated sodium channel Nav1.6 in rats. Neuropharmacology. 153:111–20. DOI: 10.1016/j.neuropharm.2019.04.024. PMID: 31054938. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065535209&origin=inward.
Article10. Matak I, Riederer P, Lacković Z. 2012; Botulinum toxin's axonal transport from periphery to the spinal cord. Neurochem Int. 61:236–9. DOI: 10.1016/j.neuint.2012.05.001. PMID: 22580329. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863449492&origin=inward.
Article11. Bu HL, Xia YZ, Liu PM, Guo HM, Yuan C, Fan XC, et al. 2019; The roles of chemokine CXCL13 in the development of bone cancer pain and the regulation of morphine analgesia in rats. Neuroscience. 406:62–72. DOI: 10.1016/j.neuroscience.2019.02.025. PMID: 30826523. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85062966103&origin=inward.
Article12. Ford A, Castonguay A, Cottet M, Little JW, Chen Z, Symons-Liguori AM, et al. 2015; Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci. 35:6057–67. Erratum in: J Neurosci 2015; 35: 8971. DOI: 10.1523/JNEUROSCI.4495-14.2015. PMID: 25878279. PMCID: PMC4397603. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929243393&origin=inward.13. Bennett GJ, Xie YK. 1988; A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33:87–107. DOI: 10.1016/0304-3959(88)90209-6. PMID: 2837713. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023895875&origin=inward.
Article14. Dilley A, Bove GM. 2008; Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol. 586:593–604. DOI: 10.1113/jphysiol.2007.144105. PMID: 18006580. PMCID: PMC2375581. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=38149084163&origin=inward.
Article15. Akaike N, Shin MC, Wakita M, Torii Y, Harakawa T, Ginnaga A, et al. 2013; Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J Physiol. 591:1031–43. DOI: 10.1113/jphysiol.2012.242131. PMID: 23109108. PMCID: PMC3591713. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84873738735&origin=inward.
Article16. Zychowska M, Rojewska E, Makuch W, Luvisetto S, Pavone F, Marinelli S, et al. 2016; Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur J Pharmacol. 791:377–88. DOI: 10.1016/j.ejphar.2016.09.019. PMID: 27619001. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84987918398&origin=inward.
Article17. Curtis R, Tonra JR, Stark JL, Adryan KM, Park JS, Cliffer KD, et al. 1998; Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol Cell Neurosci. 12:105–18. DOI: 10.1006/mcne.1998.0704. PMID: 9790733. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031727821&origin=inward.
Article18. Myers RR, Shubayev VI. 2011; The ology of neuropathy: an integrative review of the role of neuroinflammation and TNF-α axonal transport in neuropathic pain. J Peripher Nerv Syst. 16:277–86. DOI: 10.1111/j.1529-8027.2011.00362.x. PMID: 22176142. PMCID: PMC4444223. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=83755184177&origin=inward.
Article19. Sung YJ, Chiu DTW, Ambron RT. 2006; Activation and retrograde transport of protein kinase G in rat nociceptive neurons after nerve injury and inflammation. Neuroscience. 141:697–709. DOI: 10.1016/j.neuroscience.2006.04.033. PMID: 16730916. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746001390&origin=inward.
Article20. Reaux-Le Goazigo A, Rivat C, Kitabgi P, Pohl M, Melik Parsadaniantz S. 2012; Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci. 36:2619–31. DOI: 10.1111/j.1460-9568.2012.08179.x. PMID: 22694179. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84865865940&origin=inward.
Article21. Laganà M, Schlecht-Louf G, Bachelerie F. 2021; The G protein-coupled receptor kinases (GRKs) in chemokine receptor-mediated immune cell migration: from molecular cues to physiopathology. Cells. 10:75. DOI: 10.3390/cells10010075. PMID: 33466410. PMCID: PMC7824814. PMID: 5c5a4905ae9a4538882747e916612998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100226063&origin=inward.
Article22. Schioppa T, Sozio F, Barbazza I, Scutera S, Bosisio D, Sozzani S, et al. 2020; Molecular basis for CCRL2 regulation of leukocyte migration. Front Cell Dev Biol. 8:615031. DOI: 10.3389/fcell.2020.615031. PMID: 33363177. PMCID: PMC7758318. PMID: 99ae512bd2604cdbae7654e1378ccbc7. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098201685&origin=inward.
Article23. Jiang BC, Liu T, Gao YJ. 2020; Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 212:107581. DOI: 10.1016/j.pharmthera.2020.107581. PMID: 32450191. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085585010&origin=inward.
Article24. Lau S, Feitzinger A, Venkiteswaran G, Wang J, Lewellis SW, Koplinski CA, et al. 2020; A negative-feedback loop maintains optimal chemokine concentrations for directional cell migration. Nat Cell Biol. 22:266–73. DOI: 10.1038/s41556-020-0465-4. PMID: 32042179. PMCID: PMC7809593. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079456516&origin=inward.
Article25. Proske U, Luff AR. 1998; Mechanical sensitivity of muscle afferents in a nerve treated with colchicine. Exp Brain Res. 119:391–8. DOI: 10.1007/s002210050354. PMID: 9551839. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031932974&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Botulinum Toxin in Pain Management
- Botulinum Toxin-Type A in Cervical Myofascial Pain Syndrome: A report of 3 cases
- The Effect of Cervical Stellate Ganglion Block Using Botulinum Toxin Type A in Intractable Cancer-related Pain: Case Report
- Botulinum Toxin Type A Injection for Neuropathic Pain in a Patient With a Brain Tumor: A Case Report
- Botulinum toxin type A therapy in cluster headache: A case report