J Rheum Dis.
2022 Oct;29(4):254-260. 10.4078/jrd.21.0046.
Adenosine Deaminase 2 Deficiency Caused by Biallele Variants Including Splicing Variant: The First Case in Korea
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Hospital, Seoul, Korea
- 2GENOME INSIGHT Inc., Korea
- 3Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- 4Hospital Medicine Center, Seoul National University Hospital, Seoul, Korea
- KMID: 2533626
- DOI: http://doi.org/10.4078/jrd.21.0046
Abstract
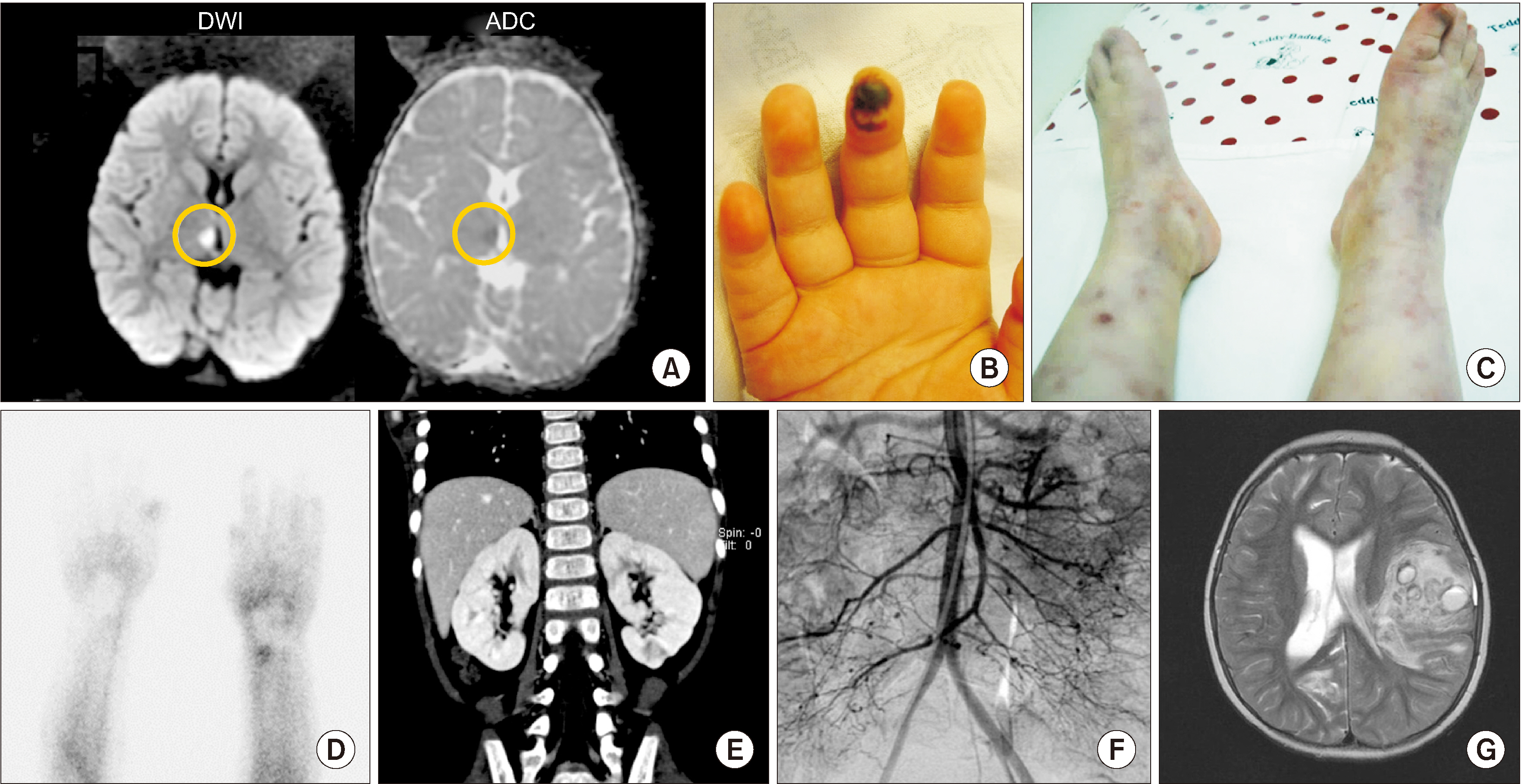
- Deficiency of adenosine deaminase 2 (DADA2) is an autoinflammatory disease caused by pathogenic variants of the ADA2 gene and has similar clinical features to polyarteritis nodosa (PAN). We, herein, report a case of DADA2 in Korea that was diagnosed in a patient with childhood-onset PAN. The patient had a truncal ataxia and facial palsy caused by thalamic infarction at 34 months of age. Livedo reticularis with Raynaud phenomenon and abdominal pain with fever were followed. Radiologic examination showed multiple infarctions in brain and kidney. She was diagnosed with PAN using skin biopsy and angiography. She had severe hemorrhagic strokes despite medical treatments. Her disease activity was controlled after adding a tumor necrosis factor-α inhibitor. Molecular analysis revealed compound heterozygous pathogenic variants of ADA2 gene. This is the first case of DADA2 in Korea. Genetic analysis for ADA2 gene should be considered in patients with childhood-onset PAN.
Figure
Reference
-
1. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2013; 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65:1–11. DOI: 10.1002/art.37715. PMID: 23045170.
Article2. Navon Elkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. 2014; Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 370:921–31. DOI: 10.1056/NEJMoa1307362. PMID: 24552285.
Article3. Iudici M, Quartier P, Pagnoux C, Merlin E, Agard C, Aouba A, et al. 2018; Childhood- versus adult-onset polyarteritis nodosa results from the French Vasculitis Study Group registry. Autoimmun Rev. 17:984–9. DOI: 10.1016/j.autrev.2018.08.001. PMID: 30114520.
Article4. Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. 2014; Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 370:911–20. DOI: 10.1056/NEJMoa1307361. PMID: 24552284. PMCID: PMC4193683.5. Meyts I, Aksentijevich I. 2018; Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J Clin Immunol. 38:569–78. DOI: 10.1007/s10875-018-0525-8. PMID: 29951947. PMCID: PMC6061100.
Article6. Lee PY. 2018; Vasculopathy, immunodeficiency, and bone marrow failure: the intriguing syndrome caused by deficiency of adenosine deaminase 2. Front Pediatr. 6:282. DOI: 10.3389/fped.2018.00282. PMID: 30406060. PMCID: PMC6200955.
Article7. Pinto B, Deo P, Sharma S, Syal A, Sharma A. 2021; Expanding spectrum of DADA2: a review of phenotypes, genetics, pathogenesis and treatment. Clin Rheumatol. 40:3883–96. DOI: 10.1007/s10067-021-05711-w. PMID: 33791889.
Article8. Human A, Pagnoux C. 2019; Diagnosis and management of ADA2 deficient polyarteritis nodosa. Int J Rheum Dis. 22 Suppl 1:69–77. DOI: 10.1111/1756-185X.13283. PMID: 29624883.
Article9. Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. 1990; The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 33:1088–93. DOI: 10.1002/art.1780330805. PMID: 1975174.
Article10. Westendorp WF, Nederkoorn PJ, Aksentijevich I, Hak AE, Lichtenbelt KD, Braun KP. 2015; Unexplained early-onset lacunar stroke and inflammatory skin lesions: consider ADA2 deficiency. Neurology. 84:2092–3. DOI: 10.1212/WNL.0000000000001581. PMID: 25888558. PMCID: PMC4442100.
Article11. Ombrello AK, Qin J, Hoffmann PM, Kumar P, Stone D, Jones A, et al. 2019; Treatment strategies for deficiency of adenosine deaminase 2. N Engl J Med. 380:1582–4. DOI: 10.1056/NEJMc1801927. PMID: 30995379. PMCID: PMC7372950.
Article12. Chung SA, Gorelik M, Langford CA, Maz M, Abril A, Guyatt G, et al. 2021; 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of polyarteritis nodosa. Arthritis Rheumatol. 73:1384–93. DOI: 10.1002/art.41776. PMID: 34235883.
Article13. Huang Z, Li T, Nigrovic PA, Lee PY. 2020; Polyarteritis nodosa and deficiency of adenosine deaminase 2 - shared genealogy, generations apart. Clin Immunol. 215:108411. DOI: 10.1016/j.clim.2020.108411. PMID: 32276138. PMCID: PMC7387119.
Article14. Caorsi R, Penco F, Schena F, Gattorno M. 2016; Monogenic polyarteritis: the lesson of ADA2 deficiency. Pediatr Rheumatol Online J. 14:51. DOI: 10.1186/s12969-016-0111-7. PMID: 27609179. PMCID: PMC5015262.
Article15. Gibson KM, Morishita KA, Dancey P, Moorehead P, Drögemöller B, Han X, et al. 2019; Identification of novel adenosine deaminase 2 gene variants and varied clinical phenotype in pediatric vasculitis. Arthritis Rheumatol. 71:1747–55. DOI: 10.1002/art.40913. PMID: 31008556.
Article16. Rama M, Duflos C, Melki I, Bessis D, Bonhomme A, Martin H, et al. 2018; A decision tree for the genetic diagnosis of deficiency of adenosine deaminase 2 (DADA2): a French reference centres experience. Eur J Hum Genet. 26:960–71. DOI: 10.1038/s41431-018-0130-6. PMID: 29681619. PMCID: PMC6018671.
Article17. Baralle D, Baralle M. 2005; Splicing in action: assessing disease causing sequence changes. J Med Genet. 42:737–48. DOI: 10.1136/jmg.2004.029538. PMID: 16199547. PMCID: PMC1735933.
Article18. Cartegni L, Chew SL, Krainer AR. 2002; Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet. 3:285–98. DOI: 10.1038/nrg775. PMID: 11967553.
Article19. Crotti LB, Horowitz DS. 2009; Exon sequences at the splice junctions affect splicing fidelity and alternative splicing. Proc Natl Acad Sci U S A. 106:18954–9. DOI: 10.1073/pnas.0907948106. PMID: 19855008. PMCID: PMC2776460.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenosine Deaminase Activities in Sera and Erythrocytes of Patients with Psoriasis
- Diagnostic value of adenosine deaminase and lysozyme activity in pleural effusion
- A study on the diagnostic value of cerebrospinal fluid adenosine deaminase activity in children with tuberculous meningitis
- Adenosine deaminase activity in bronchoalveolar lavage fluid in patients with pulmonary tuberculosis
- Adenosine Deaminase Activities in Sera and Erythrocytes of Leprosy Patients