Anat Cell Biol.
2022 Sep;55(3):341-355. 10.5115/acb.22.013.
Comparative histological study on the effect of tramadol abuse on the testis of juvenile and adult male albino mice
- Affiliations
-
- 1Department of Histology and Cell Biology, Faculty of Medicine, Assiut University, Assiut, Egypt
- 2Department of Histology and Cell Biology, Sphinx University, New Assiut City, Egypt
- 3Department of Anatomy and Embriology, Faculty of Medicine, Assiut University, Assiut, Egypt
- 4Department of Histology and Cell Biology, Faculty of Medicine, Aswan University, Aswan, Egypt
- 5Department of Histology and Cell Biology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt
- KMID: 2533360
- DOI: http://doi.org/10.5115/acb.22.013
Abstract
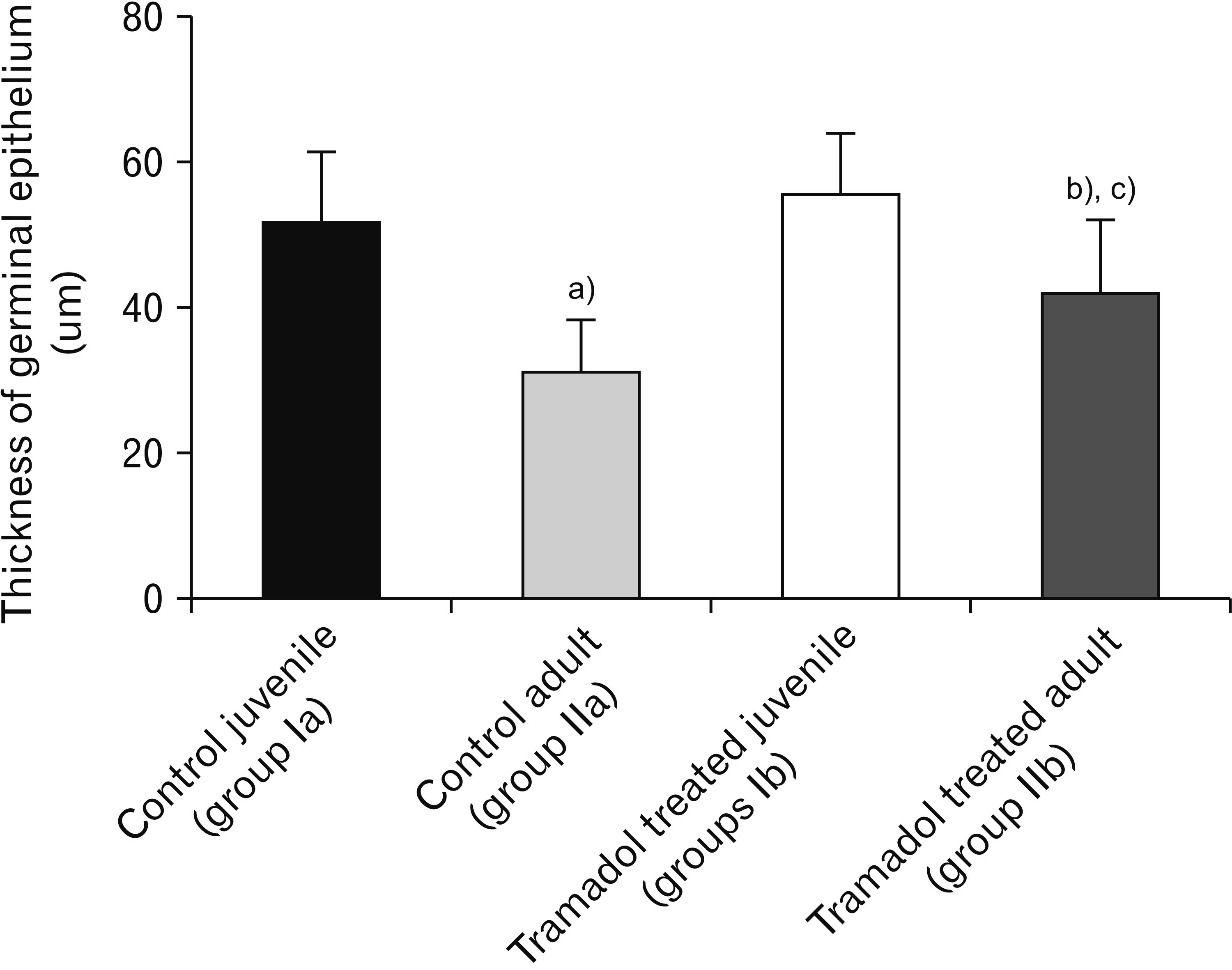
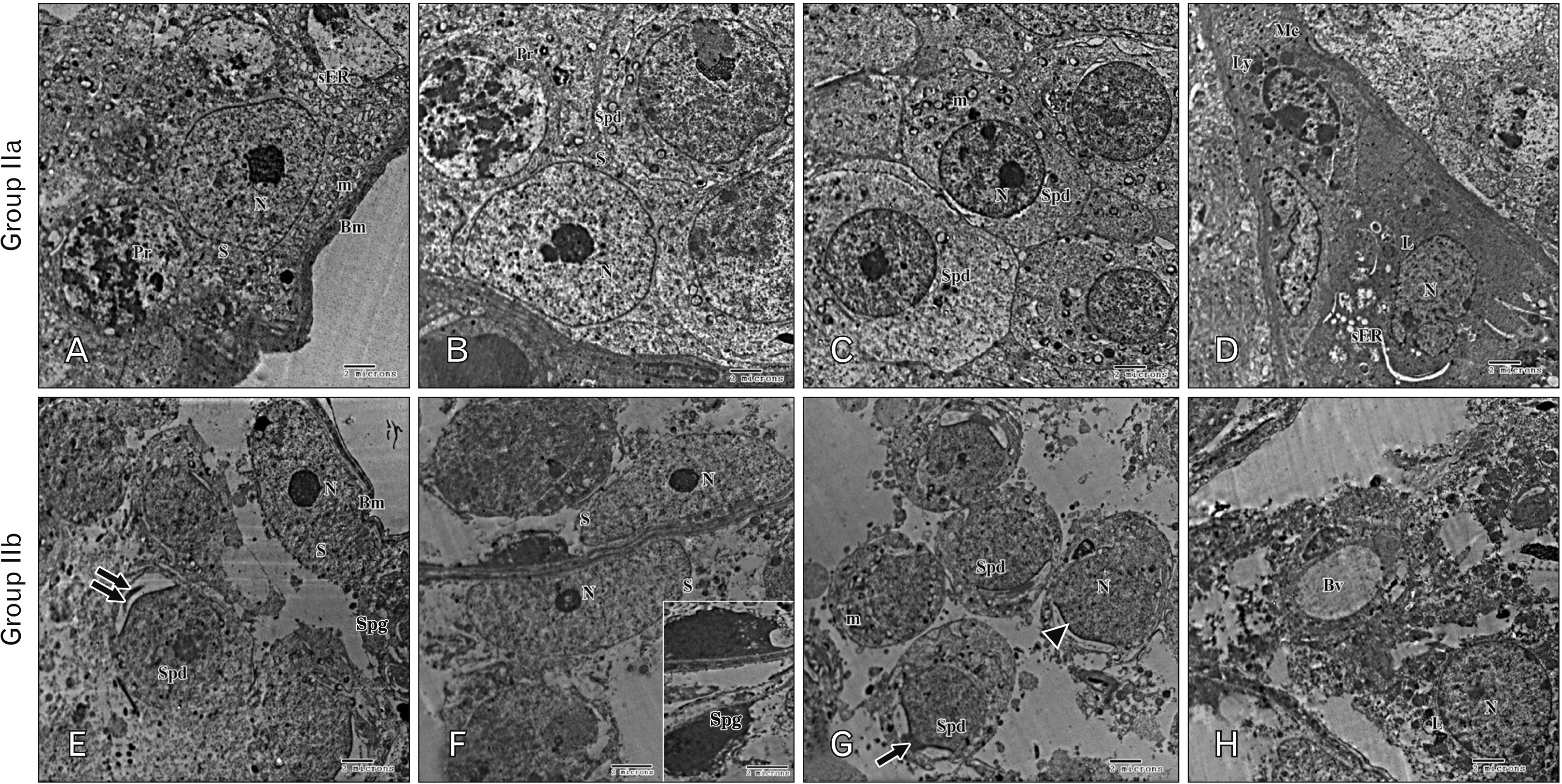
- As a synthetic analog of codeine, tramadol is often prescribed to treat mild to moderate pains. This study was designed to estimate and compare the histological effect of tramadol on testes of both juvenile and adult male albino mice. A total number of 40 healthy male albino mice were classified into two main groups as follows: group I (juvenile group, includes 20 mice aged three weeks) subdivided equally into group Ia (control group received isotonic saline) and group Ib (tramadoltreated group received 40 mg/kg/d tramadol orally for 30 days); group II (adult group, includes 20 mice aged two months) subdivided equally into group IIa (control group received isotonic saline) and group IIb (tramadol-treated group). Juvenile and adult tramadol-treated groups showed numerous testicular changes, including blood vessels congestion, widening of intercellular spaces, vacuolization in interstitial tissues, luminal germ cells exfoliation, and increased expression of caspase-3 that indicated cellular apoptosis. In the ultrastructural examination, spermatogenic cells degenerated with the frequent appearance of apoptotic cells. Sertoli cells showed vacuolations, large lipid droplets, and disrupted intercellular cell junctions. These observed testicular changes were markedly observed in the juvenile group. Testicular abnormalities and apoptotic changes can be caused by tramadol administration. These abnormalities are more common in juvenile mice.
Keyword
Figure
Reference
-
References
1. Candeletti S, Lopetuso G, Cannarsa R, Cavina C, Romualdi P. 2006; Effects of prolonged treatment with the opiate tramadol on prodynorphin gene expression in rat CNS. J Mol Neurosci. 30:341–7. DOI: 10.1385/JMN:30:3:341. PMID: 17401159.
Article2. Hawton K, Bergen H, Simkin S, Wells C, Kapur N, Gunnell D. 2012; Six-year follow-up of impact of co-proxamol withdrawal in England and Wales on prescribing and deaths: time-series study. PLoS Med. 9:e1001213. DOI: 10.1371/journal.pmed.1001213. PMID: 22589703. PMCID: PMC3348153. PMID: 77833b219ee74089a2b3311167cc468e.
Article3. Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. 2017; Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg. 124:44–51. DOI: 10.1213/ANE.0000000000001683. PMID: 27861439.4. Leppert W, Łuczak J. 2005; The role of tramadol in cancer pain treatment--a review. Support Care Cancer. 13:5–17. DOI: 10.1007/s00520-004-0720-4. PMID: 15668743.
Article5. Grond S, Sablotzki A. 2004; Clinical pharmacology of tramadol. Clin Pharmacokinet. 43:879–923. DOI: 10.2165/00003088-200443130-00004. PMID: 15509185.
Article6. Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. 2013; Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 129:116–24. DOI: 10.1016/j.drugalcdep.2012.09.018. PMID: 23098678. PMCID: PMC3594406.
Article7. Scott LJ, Perry CM. 2000; Tramadol: a review of its use in perioperative pain. Drugs. 60:139–76. DOI: 10.2165/00003495-200060010-00008. PMID: 10929933.8. Aloisi AM, Ceccarelli I, Fiorenzani P, Maddalena M, Rossi A, Tomei V, Sorda G, Danielli B, Rovini M, Cappelli A, Anzini M, Giordano A. 2010; Aromatase and 5-alpha reductase gene expression: modulation by pain and morphine treatment in male rats. Mol Pain. 6:69. DOI: 10.1186/1744-8069-6-69. PMID: 20977699. PMCID: PMC2978140.
Article9. Ghoneim FM, Khalaf HA, Elsamanoudy AZ, Helaly AN. 2014; Effect of chronic usage of tramadol on motor cerebral cortex and testicular tissues of adult male albino rats and the effect of its withdrawal: histological, immunohistochemical and biochemical study. Int J Clin Exp Pathol. 7:7323–41. PMID: 25550769. PMCID: PMC4270590.10. Bar-Or D, Salottolo KM, Orlando A, Winkler JV. Tramadol ODT Study Group. 2012; A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol. 61:736–43. DOI: 10.1016/j.eururo.2011.08.039. PMID: 21889833.
Article11. Creasy DM. 2002; Histopathology of the male reproductive system I: techniques. Curr Protoc Toxicol. Chapter 16:Unit16.3. DOI: 10.1002/0471140856.tx1603s12. PMID: 20963755.
Article12. Bancroft JD, Layton C. Suvarna SK, Layton C, Bancroft JD, editors. 2013. The hematoxylins and eosin. Bancroft's Theory and Practice of Histological Techniques. 7th ed. Churchill Livingstone of Elsevier;Philadelphia: p. 173–86. DOI: 10.1016/B978-0-7020-4226-3.00010-X.
Article13. Glauert AM, Lewis PR. 2014. Biological specimen preparation for transmission electron microscopy. Princeton University Press;Princeton: DOI: 10.1002/0471140856.tx1603s12.14. O'Callaghan JP, Jensen KF. 1992; Enhanced expression of glial fibrillary acidic protein and the cupric silver degeneration reaction can be used as sensitive and early indicators of neurotoxicity. Neurotoxicology. 13:113–22. PMID: 1508411.15. Martyn-St James M, Cooper K, Kaltenthaler E, Dickinson K, Cantrell A, Wylie K, Frodsham L, Hood C. 2015; Tramadol for premature ejaculation: a systematic review and meta-analysis. BMC Urol. 15:6. DOI: 10.1186/1471-2490-15-6. PMID: 25636495. PMCID: PMC4417346.
Article16. Ahmed AI, El-Dawy K, Fawzy MM, Abdallah HA, Elsaid HN, Elmesslamy WO. 2018; Retrospective review of tramadol abuse. Slov Vet Res. 55(Suppl 20):471–83.17. Abdellatief RB, Elgamal DA, Mohamed EE. 2015; Effects of chronic tramadol administration on testicular tissue in rats: an experimental study. Andrologia. 47:674–9. DOI: 10.1111/and.12316. PMID: 25228095.
Article18. Cerilli LA, Kuang W, Rogers D. 2010; A practical approach to testicular biopsy interpretation for male infertility. Arch Pathol Lab Med. 134:1197–204. DOI: 10.5858/2009-0379-RA.1. PMID: 20670143.
Article19. Salama N, Bergh A, Damber JE. 2003; The changes in testicular vascular permeability during progression of the experimental varicocele. Eur Urol. 43:84–91. DOI: 10.1016/S0302-2838(02)00501-8. PMID: 12507549.
Article20. Awadalla EA, Salah-Eldin A. 2005; Histopathological and molecular studies on tramadol mediated hepato-renal toxicity in rats. J Pharm Biol Sci. 10:90–102.21. Essam Hafez M, Sahar Issa Y, Safaa Abdel Rahman M. 2015; Parenchymatous toxicity of tramadol: histopathological and biochemical study. J Alcohol Drug Depend. 3:225. DOI: 10.4172/2329-6488.1000225.
Article22. Elkhateeb A, El Khishin I, Megahed O, Mazen F. 2015; Effect of Nigella sativa Linn oil on tramadol-induced hepato- and nephrotoxicity in adult male albino rats. Toxicol Rep. 2:512–9. DOI: 10.1016/j.toxrep.2015.03.002. PMID: 28962386. PMCID: PMC5598165.
Article23. Mohamed D, Saber A, Omar A, Soliman A. 2014; Effect of cadmium on the testes of adult albino rats and the ameliorating effect of zinc and vitamin E. Br J Sci. 11:72–95.24. Nolte T, Harleman JH, Jahn W. 1995; Histopathology of chemically induced testicular atrophy in rats. Exp Toxicol Pathol. 47:267–86. DOI: 10.1016/S0940-2993(11)80260-5. PMID: 8855122.
Article25. Caju FM, Queiroz GCD, Torres SM, Tenório BM, Júnior VAS. 2011; Opioid system manipulation during testicular development: results on sperm production and sertoli cells population. Acta Sci Biol Sci. 33:219–25. DOI: 10.4025/actascibiolsci.v33i2.5940. PMID: 9379367d9bf24995aa270c4eff76ad63.26. Ahmed MA, Kurkar A. 2014; Effects of opioid (tramadol) treatment on testicular functions in adult male rats: the role of nitric oxide and oxidative stress. Clin Exp Pharmacol Physiol. 41:317–23. DOI: 10.1111/1440-1681.12213. PMID: 24472030.
Article27. Zhang YT, Zheng QS, Pan J, Zheng RL. 2004; Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol. 95:53–8. DOI: 10.1111/j.1742-7843.2004.950202.x. PMID: 15379780.
Article28. Berruti G. 2006; Signaling events during male germ cell differentiation: update, 2006. Front Biosci. 11:2144–56. DOI: 10.2741/1957. PMID: 16720301.
Article29. Lombardo F, Sgrò P, Salacone P, Gilio B, Gandini L, Dondero F, Jannini EA, Lenzi A. 2005; Androgens and fertility. J Endocrinol Invest. 28(3 Suppl):51–5. DOI: 10.1111/j.1365-2605.2005.00585.x. PMID: 16236065.30. Soliman HM, Wagih HM, Attia GM, Algaidi SA. 2014; Light and electron microscopic study on the effect of antischizophrenic drugs on the structure of seminiferous tubules of adult male albino rats. Folia Histochem Cytobiol. 52:335–49. DOI: 10.5603/FHC.a2014.0038. PMID: 25535927.
Article31. Heidari Z, Mahmoudzadeh-Sagheb H, Kohan F. 2012; A quantitative and qualitative study of rat testis following administration of methadone and buprenorphine. Int J High Risk Behav Addict. 1:14–7. DOI: 10.5812/ijhrba.4119.
Article32. Sorge RE, Stewart J. 2006; The effects of long-term chronic buprenorphine treatment on the locomotor and nucleus accumbens dopamine response to acute heroin and cocaine in rats. Pharmacol Biochem Behav. 84:300–5. DOI: 10.1016/j.pbb.2006.05.013. PMID: 16806444.
Article33. Rashed RMA, Mohamed IK, El-Alfy SH. 2010; Effects of two different doses of melatonin on the spermatogenic cells of rat testes: a light and electron microscopic study. Egypt J Histol. 33:819–35.34. Manivannan B, Mittal R, Goyal S, Ansari AS, Lohiya NK. 2009; Sperm characteristics and ultrastructure of testes of rats after long-term treatment with the methanol subfraction of Carica papaya seeds. Asian J Androl. 11:583–99. DOI: 10.1038/aja.2009.25. PMID: 19648937. PMCID: PMC3735006.
Article35. Jezek D, Simunić-Banek L, Pezerović-Panijan R. 1993; Effects of high doses of testosterone propionate and testosterone enanthate on rat seminiferous tubules--a stereological and cytological study. Arch Toxicol. 67:131–40. DOI: 10.1007/BF01973684. PMID: 8481101.36. Sharpe RM, McKinnell C, Kivlin C, Fisher JS. 2003; Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 125:769–84. DOI: 10.1530/rep.0.1250769. PMID: 12773099.
Article37. Mohamed HM, Mahmoud AM. 2019; Chronic exposure to the opioid tramadol induces oxidative damage, inflammation and apoptosis, and alters cerebral monoamine neurotransmitters in rats. Biomed Pharmacother. 110:239–47. DOI: 10.1016/j.biopha.2018.11.141. PMID: 30508735.
Article38. Drobnis EZ, Nangia AK. 2017; Pain medications and male reproduction. Adv Exp Med Biol. 1034:39–57. DOI: 10.1007/978-3-319-69535-8_6. PMID: 29256126.
Article39. Erkkilä K, Aito H, Aalto K, Pentikäinen V, Dunkel L. 2002; Lactate inhibits germ cell apoptosis in the human testis. Mol Hum Reprod. 8:109–17. DOI: 10.1093/molehr/8.2.109. PMID: 11818513.
Article40. Eddy EM. 1999; Role of heat shock protein HSP70-2 in spermatogenesis. Rev Reprod. 4:23–30. DOI: 10.1530/ror.0.0040023. PMID: 10051099.
Article41. Newton AP, Cadena SM, Rocha ME, Carnieri EG, Martinelli de Oliveira MB. 2005; Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett. 160:49–59. DOI: 10.1016/j.toxlet.2005.06.004. PMID: 16023799.
Article42. Tamura I, Kanbara Y, Saito M, Horimoto K, Satoh M, Yamamoto H, Oyama Y. 2012; Triclosan, an antibacterial agent, increases intracellular Zn(2+) concentration in rat thymocytes: its relation to oxidative stress. Chemosphere. 86:70–5. DOI: 10.1016/j.chemosphere.2011.09.009. PMID: 22000841.
Article43. Abdel-Zaher AO, Abdel-Rahman MS, Elwasei FM. 2011; Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology. 32:725–33. DOI: 10.1016/j.neuro.2011.08.001. PMID: 21855572.
Article44. Halawa AM. 2010; Effect of sildenafil administration on ischemic reperfusion of the testis in adult albino rat light and electron microscopic study. Egypt J Histol. 2:380–95.45. Gouda ZA, Selim AO. 2013; A possible correlation between the testicular structure and short photoperiod exposure in young albino rats: light and electron microscopic study. Egypt J Histol. 36:28–38. DOI: 10.1097/01.EHX.0000423980.95382.0c.
Article46. Bassiony MM, Youssef UM, El-Gohari H. 2020; Free testosterone and prolactin levels and sperm morphology and function among male patients with tramadol abuse: a case-control study. J Clin Psychopharmacol. 40:405–8. DOI: 10.1097/JCP.0000000000001223. PMID: 32639294.
Article47. Popovic M, Janicijevic-Hudomal S, Kaurinovic B, Rasic J, Trivic S, Vojnović M. 2009; Antioxidant effects of some drugs on immobilization stress combined with cold restraint stress. Molecules. 14:4505–16. DOI: 10.3390/molecules14114505. PMID: 19924083. PMCID: PMC6254731.
Article48. El-Gaafarawi II. 2006; Biochemical toxicity induced by tramadol administration in male rats. Egypt J Hosp Med. 23:353–62. DOI: 10.21608/ejhm.2006.17946.
Article