Ann Surg Treat Res.
2022 Sep;103(3):160-168. 10.4174/astr.2022.103.3.160.
Protective effects of sigma 1 receptor agonist PRE084 on 2,4,6-trinitrobenzene sulfonic acid–induced experimental colitis in mice
- Affiliations
-
- 1Department of Internal Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- 2Department of Physiology, Catholic Kwandong University College of Medicine, Gangneung, Korea
- 3Department of Surgery, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- KMID: 2532928
- DOI: http://doi.org/10.4174/astr.2022.103.3.160
Abstract
- Purpose
We aimed to investigate the protective effect of sigma 1 receptor agonist and antagonist, PRE084 and BD1047, respectively, on 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice.
Methods
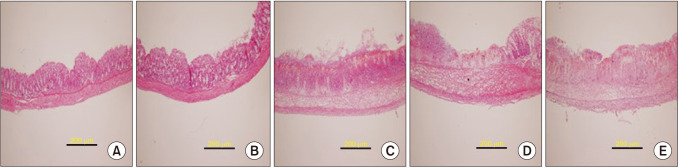
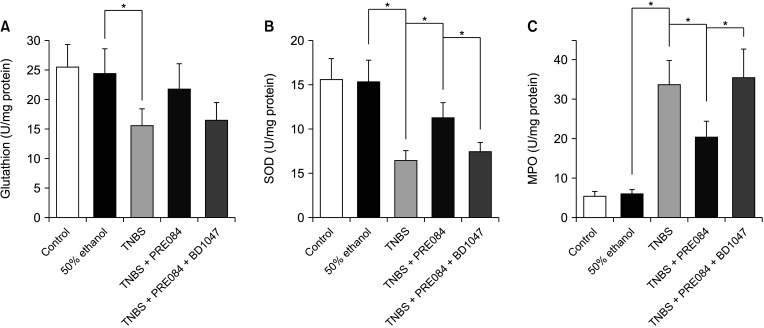
Thirty male ICR mice were randomly divided into 5 groups: control, 50% ethanol, colitis, PRE084 + colitis, and combined (PRE084 + BD1047 + colitis). Colitis was induced by intrarectal administration of TNBS. PRE084 and BD1047 were injected daily, starting 3 days before colitis induction. Distal colon tissue was excised for histopathological evaluation, and levels of glutathione (GSH), superoxide dismutase (SOD), myeloperoxidase (MPO), and lipid peroxidation were determined.
Results
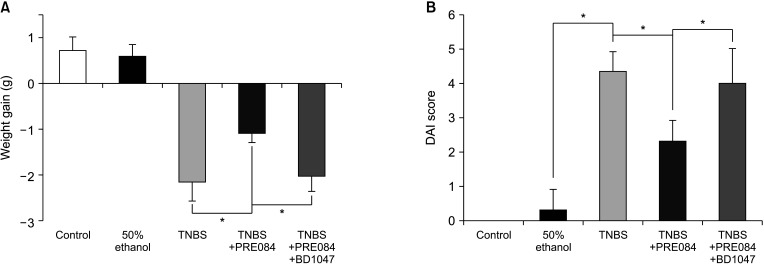
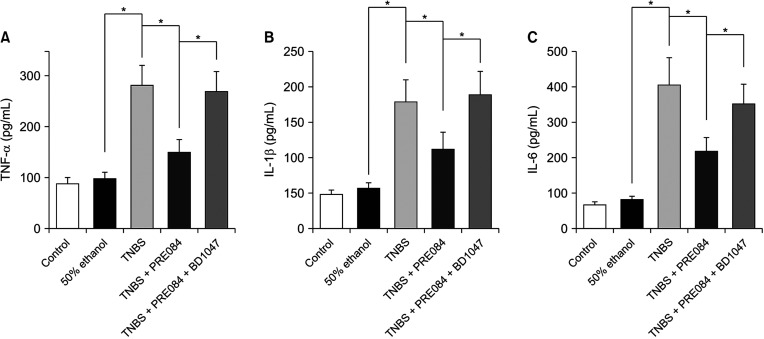
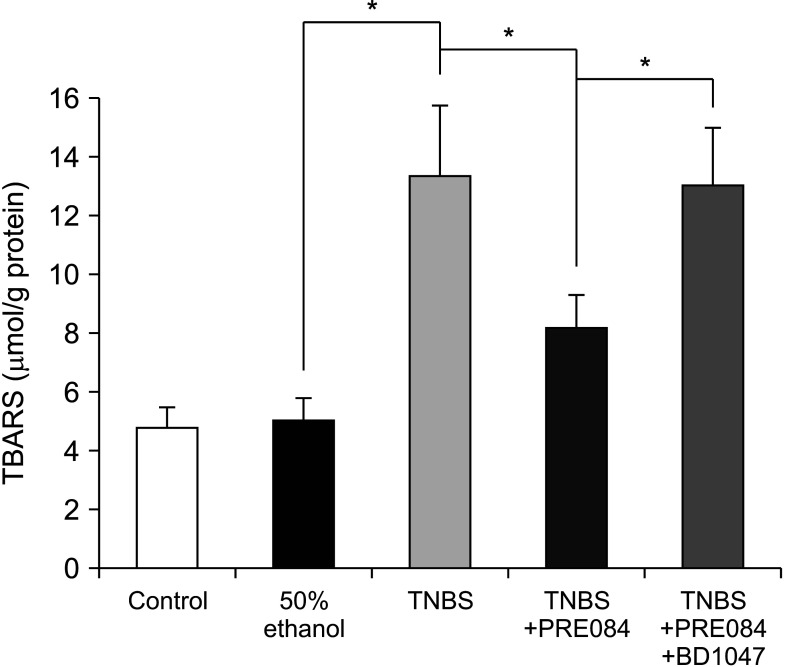
Colitis caused weight loss, mucosal damage, upregulation of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, MPO, and thiobarbituric acid reactive substance activities, and downregulation of GSH and SOD activities. These changes caused by TNBS-induced colitis were significantly ameliorated by PRE084 pretreatment. However, the combined pretreatment with BD1047 significantly attenuated the protective effect of PRE084, thereby reverting to the colitis-induced state.
Conclusion
We conclude that the sigma 1 receptor agonist PRE084 exhibits significant protective effects against TNBSinduced colitis, which appears to be at least partly mediated by the inhibition of inflammation and oxidative stress, and enhancement of antioxidant properties. Collectively, these results suggest that PRE084 might be an effective drug for the treatment of ulcerative colitis.
Keyword
Figure
Reference
-
1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007; 369:1641–1657. PMID: 17499606.
Article2. Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011; 63:629–642. PMID: 21857074.
Article3. Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013; 29:357–362. PMID: 23695429.
Article4. Kwak MS, Cha JM, Lee HH, Choi YS, Seo SI, Ko KJ, et al. Emerging trends of inflammatory bowel disease in South Korea: a nationwide population-based study. J Gastroenterol Hepatol. 2019; 34:1018–1026. PMID: 30447025.
Article5. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380:1606–1619. PMID: 22914296.
Article6. Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018; 53:379–389. PMID: 29523023.
Article7. Hirten RP, Sands BE. New Therapeutics for ulcerative colitis. Annu Rev Med. 2021; 72:199–213. PMID: 33502898.
Article8. Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, et al. The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond). 2016; 11:9–15. PMID: 27656280.
Article9. Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol. 2015; 1:154–170. PMID: 26000334.
Article10. Özden H, Şahin Y, Kilitçi A, Karaca G, Gömeç M, Yildiz A, et al. Comparison of the healing effects of mesazaline and Ganoderma lucidum in acetic acid-induced colitis in rats. Ann Surg Treat Res. 2022; 102:29–35. PMID: 35071117.
Article11. Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022; 21:103017. PMID: 34902606.
Article12. Patlevič P, Vašková J, Švorc P Jr, Vaško L, Švorc P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr Med Res. 2016; 5:250–258. PMID: 28462126.
Article13. Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019; 13:772–784. PMID: 30715224.
Article14. Pravda J. Hydrogen peroxide and disease: towards a unified system of pathogenesis and therapeutics. Mol Med. 2020; 26:41. PMID: 32380940.
Article15. Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013; 19:1711–1747. PMID: 23305298.
Article16. Wendland BE, Aghdassi E, Tam C, Carrrier J, Steinhart AH, Wolman SL, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr. 2001; 74:259–264. PMID: 11470730.
Article17. Santhanam S, Venkatraman A, Ramakrishna BS. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut. 2007; 56:1543–1549. PMID: 17483192.
Article18. Nguyen L, Lucke-Wold BP, Mookerjee S, Kaushal N, Matsumoto RR. Sigma-1 receptors and neurodegenerative diseases: towards a hypothesis of Sigma-1 receptors as amplifiers of neurodegeneration and neuroprotection. Adv Exp Med Biol. 2017; 964:133–152. PMID: 28315269.
Article19. Gris G, Cobos EJ, Zamanillo D, Portillo-Salido E. Sigma-1 receptor and inflammatory pain. Inflamm Res. 2015; 64:377–381. PMID: 25902777.
Article20. Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012; 682:12–20. PMID: 22381068.
Article21. Almási N, Török S, Valkusz Z, Tajti M, Csonka Á, Murlasits Z, et al. Sigma-1 receptor engages an anti-inflammatory and antioxidant feedback loop mediated by peroxiredoxin in experimental colitis. Antioxidants (Basel). 2020; 9:1081.
Article22. Min JK, Lee CH, Jang SE, Park JW, Lim SJ, Kim DH, et al. Amelioration of trinitrobenzene sulfonic acid-induced colitis in mice by liquiritigenin. J Gastroenterol Hepatol. 2015; 30:858–865. PMID: 25311527.
Article23. Motawe ZY, Abdelmaboud SS, Cuevas J, Breslin JW. PRE-084 as a tool to uncover potential therapeutic applications for selective sigma-1 receptor activation. Int J Biochem Cell Biol. 2020; 126:105803. PMID: 32668330.
Article24. Hara H, Tanaka K, Harada Y, Sukamoto T. Sigma receptor-mediated effects of a new antiulcer agent, KB-5492, on experimental gastric mucosal lesions and gastric alkaline secretion in rats. J Pharmacol Exp Ther. 1994; 269:799–805. PMID: 8182548.25. Facente SN, Reiersen AM, Lenze EJ, Boulware DR, Klausner JD. Fluvoxamine for the early treatment of SARS-CoV-2 infection: a review of current evidence. Drugs. 2021; 81:2081–2089. PMID: 34851510.
Article26. Almási N, Török S, Dvorácskó S, Tömböly C, Csonka Á, Baráth Z, et al. Lessons on the sigma-1 receptor in TNBS-induced rat colitis: modulation of the UCHL-1, IL-6 pathway. Int J Mol Sci. 2020; 21:4046.
Article27. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019; 50:992–1006. PMID: 30995511.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HO-1 Reduces the Severity of Trinitrobenzene Sulfonic Acid-induced Colitis through Suppression of NF-kappaB Activation
- Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats
- CoPPIX Protects against TNBS Induced Colitis Through HO-1 Induction
- A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents
- Altered Colonic Transit in TNBS-induced Experimental Colitis in Guinea Pig and Distribution of Nitric Oxide Synthase in the Colonic Wall