J Korean Med Sci.
2022 Sep;37(35):e266. 10.3346/jkms.2022.37.e266.
Incidence and Risk Factors for Totally Implantable Venous Access Device Infections in Pediatric Patients With Cancer: A Study of 25,954 Device-Days
- Affiliations
-
- 1Department of Pediatrics, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- 2Department of Pediatrics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- KMID: 2532920
- DOI: http://doi.org/10.3346/jkms.2022.37.e266
Abstract
- Background
Totally implantable venous access devices (TIVADs) are frequently used in pediatric patients with cancer owing to their multiple benefits. Despite occasional infections with TIVADs, knowledge of the incidence and risk factors is limited.
Methods
This retrospective study included pediatric patients with cancer who received TIVAD at Chungbuk National University Hospital from 2001 to 2021. We collected data on demographics, diagnosis, duration of TIVAD use, pathogens, and other risk factors.
Results
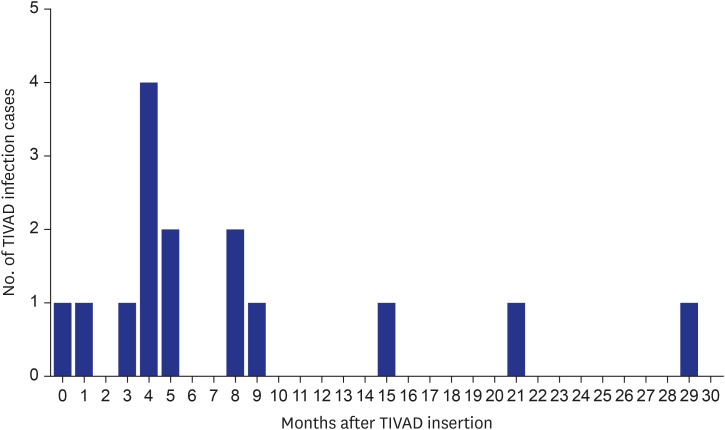
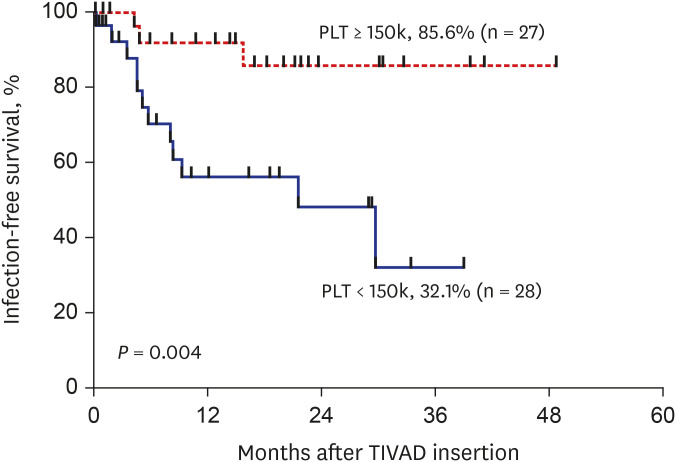
During the study period, 55 TIVADs with 25,954 device-days were applied in 49 patients. There were 15 TIVAD infections (15/55, 27.3%), with an infection rate of 0.21 infections per TIVAD per year (0.58 cases/1,000 device-days). TIVAD infections occurred at a median of 5 months (range, 8 days–30 months) after insertion. The most common causative microorganisms were methicillin-resistant coagulase-negative staphylococci (n = 8, 53.3%) followed by Escherichia coli (n = 3, 20.0%). Infection-free TIVAD survival was higher in the group with normal platelet count at insertion (platelet counts ≥ 150,000/μL) than in the group with thrombocytopenia at insertion (platelet counts < 150,000/μL) (81.3% vs. 32.1%, P = 0.004). Device removal was the mainstay of treatment (11/15, 73.3%).
Conclusion
TIVAD infection may be related to thrombocytopenia at the time of device insertion. Further studies are needed to identify preventive factors against TIVAD infections in children with cancer.
Keyword
Figure
Reference
-
1. Stovroff M, Teague WG. Intravenous access in infants and children. Pediatr Clin North Am. 1998; 45(6):1373–1393. viiiPMID: 9889758.
Article2. Jung SE, Kim YH, Jun YS, Kim DY, Park JK, Lee SC, et al. Totally implantable venous access devices in pediatric surgery patients. J Korean Surg Soc. 1997; 52(3):420–425.3. Di Carlo I, Cordio S, La Greca G, Privitera G, Russello D, Puleo S, et al. Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg. 2001; 136(9):1050–1053. PMID: 11529829.
Article4. Fratino G, Molinari AC, Parodi S, Longo S, Saracco P, Castagnola E, et al. Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 2005; 16(4):648–654. PMID: 15677621.
Article5. Alexander N. Question 3. Do Portacaths or Hickman lines have a higher risk of catheter-related bloodstream infections in children with leukaemia? Arch Dis Child. 2010; 95(3):239–241. PMID: 20308343.
Article6. Aparna S, Ramesh S, Appaji L, Srivatsa K, Shankar G, Jadhav V, et al. Complications of chemoport in children with cancer: Experience of 54,100 catheter days from a tertiary cancer center of Southern India. South Asian J Cancer. 2015; 4(3):143–145. PMID: 26942147.
Article7. Hengartner H, Berger C, Nadal D, Niggli FK, Grotzer MA. Port-A-Cath infections in children with cancer. Eur J Cancer. 2004; 40(16):2452–2458. PMID: 15519519.
Article8. Tobiansky R, Lui K, Dalton DM, Shaw P, Martin H, Isaacs D. Complications of central venous access devices in children with and without cancer. J Paediatr Child Health. 1997; 33(6):509–514. PMID: 9484682.
Article9. Ignatov A, Hoffman O, Smith B, Fahlke J, Peters B, Bischoff J, et al. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. Eur J Surg Oncol. 2009; 35(3):241–246. PMID: 18329836.
Article10. Nam SH, Kim DY, Kim SC, Kim IK. Complications and risk factors of infection in pediatric hemato-oncology patients with totally implantable access ports (TIAPs). Pediatr Blood Cancer. 2010; 54(4):546–551. PMID: 19967773.
Article11. Viana Taveira MR, Lima LS, de Araújo CC, de Mello MJ. Risk factors for central line-associated bloodstream infection in pediatric oncology patients with a totally implantable venous access port: a cohort study. Pediatr Blood Cancer. 2017; 64(2):336–342. PMID: 27666952.
Article12. Gyves J, Ensminger W, Niederhuber J, Liepman M, Cozzi E, Doan K, et al. Totally implanted system for intravenous chemotherapy in patients with cancer. Am J Med. 1982; 73(6):841–845. PMID: 6959532.
Article13. Deerojanawong J, Sawyer SM, Fink AM, Stokes KB, Robertson CF. Totally implantable venous access devices in children with cystic fibrosis: incidence and type of complications. Thorax. 1998; 53(4):285–289. PMID: 9741372.
Article14. Al-Bassam A, Al-Rabeeah A, Fouda K, Al-Ashwal A, Ozand PT. Implantable central venous access devices in children with metabolic disease. Metabolism. 1998; 47(8):900–902. PMID: 9711982.
Article15. Beck O, Muensterer O, Hofmann S, Rossmann H, Poplawski A, Faber J, et al. Central venous access devices (CVAD) in pediatric oncology patients—A single-center retrospective study over more than 9 years. Front Pediatr. 2019; 7:260. PMID: 31294007.
Article16. Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B, et al. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis. 2014; 14(2):146–159. PMID: 24314751.
Article17. Pinelli F, Cecero E, Degl’Innocenti D, Selmi V, Giua R, Villa G, et al. Infection of totally implantable venous access devices: a review of the literature. J Vasc Access. 2018; 19(3):230–242. PMID: 29512430.
Article18. Miliaraki M, Katzilakis N, Chranioti I, Stratigaki M, Koutsaki M, Psarrou M, et al. Central line-associated bloodstream infection in childhood malignancy: single-center experience. Pediatr Int. 2017; 59(7):769–775. PMID: 28376269.
Article19. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 49(1):1–45. PMID: 19489710.
Article20. Wolf J, Allison KJ, Tang L, Sun Y, Hayden RT, Flynn PM. No evidence of benefit from antibiotic lock therapy in pediatric oncology patients with central line-related bloodstream infection: results of a retrospective matched cohort study and review of the literature. Pediatr Blood Cancer. 2014; 61(10):1811–1815. PMID: 24923808.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Totally Implantable Venous Access Devices In Pediatric Surgery Patients
- Commentary on "Insertion of Totally Implantable Central Venous Access Devices by Surgeons" - What Is the Role of Surgeons When Implanting a Totally Implantable Venous Access Device to Prevent Immediate Complications?
- Pinch-Off Syndrome, a Rare Complication of Totally Implantable Venous Access Device Implantation: A Case Series and Literature Review
- A prospective study of totally implanted venous access system in 19 children with cancer
- Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case Report