Korean J Physiol Pharmacol.
2022 Sep;26(5):397-404. 10.4196/kjpp.2022.26.5.397.
Inhibition of voltage-dependent K+ channels by antimuscarinic drug fesoterodine in coronary arterial smooth muscle cells
- Affiliations
-
- 1Institute of Medical Sciences, Department of Physiology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 2Department of Medical Environmental Biology and Tropical Medicine, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 3Department of Pharmacology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 4Institute of Medical Sciences, Department of Urology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- KMID: 2532769
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.397
Abstract
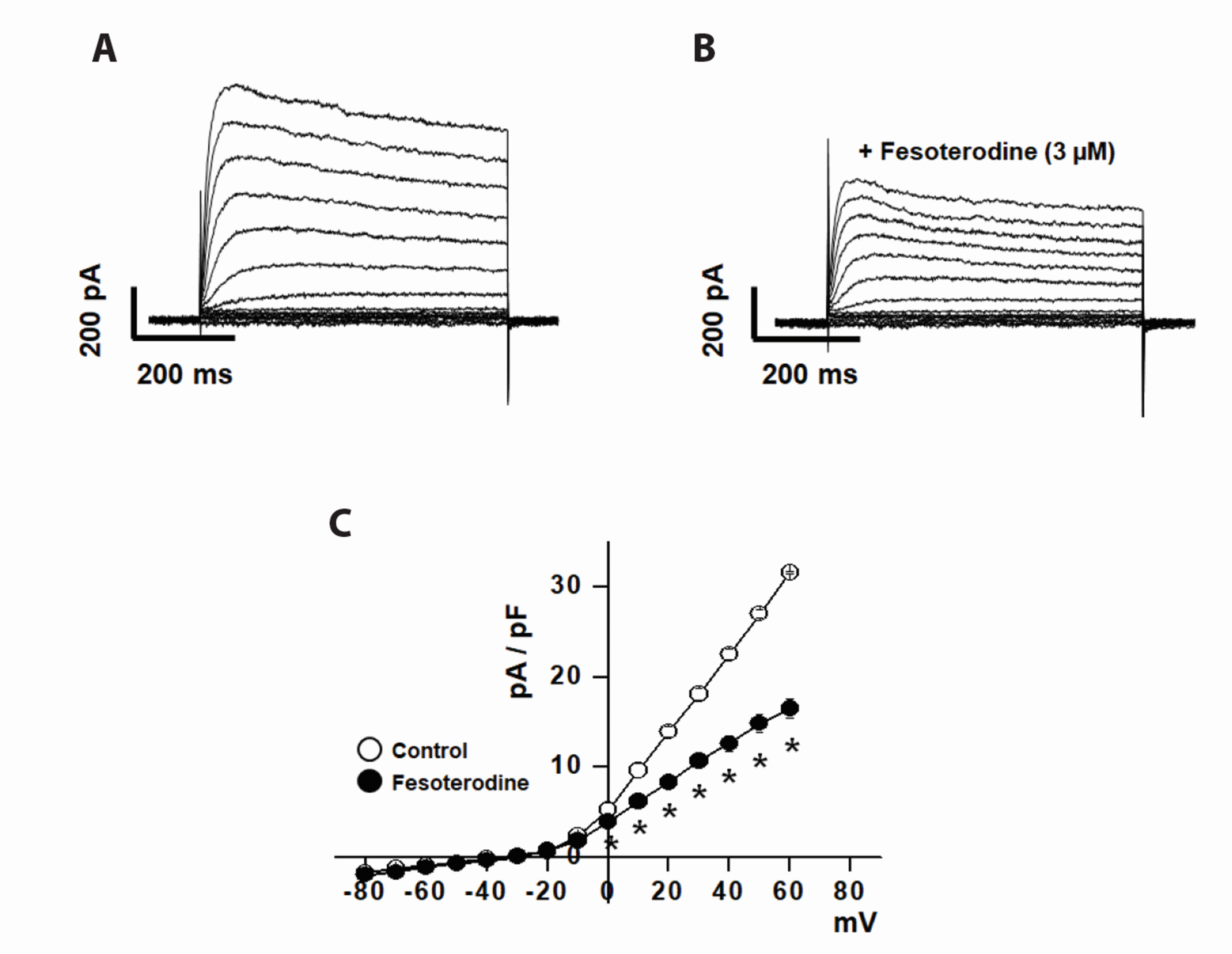
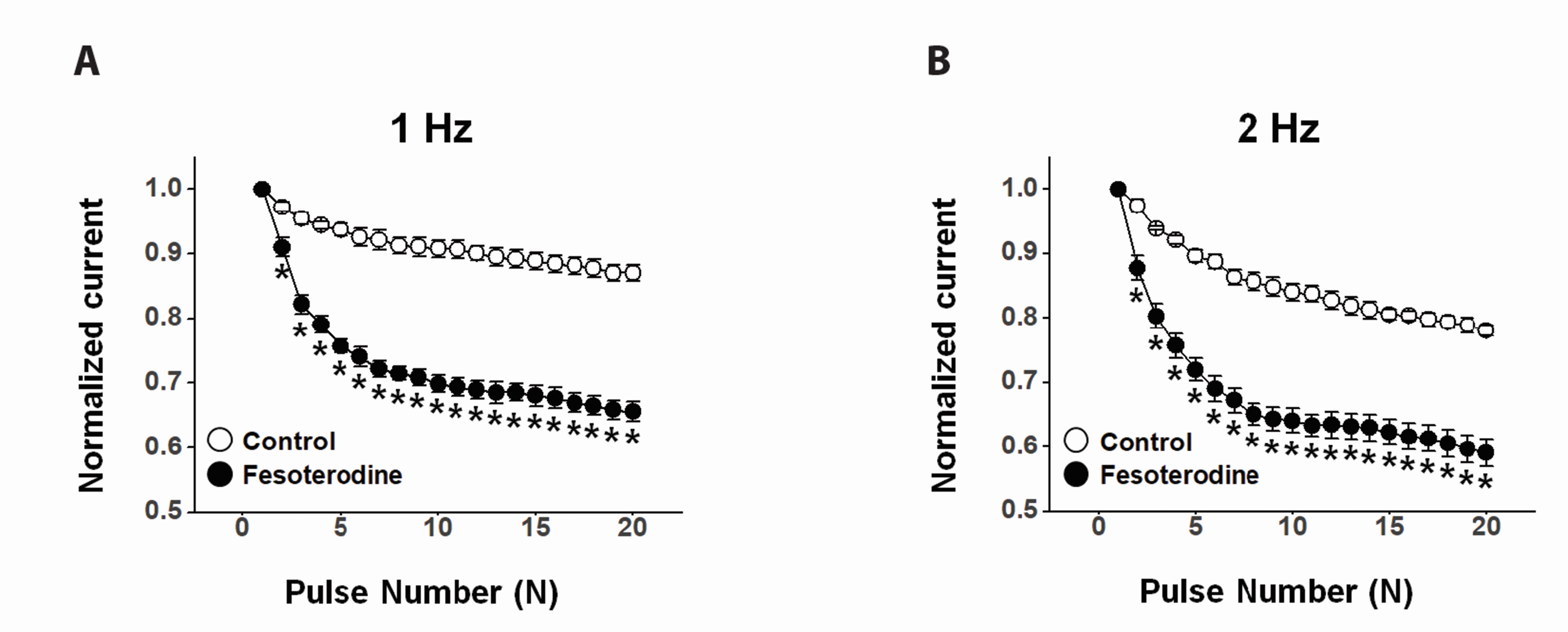
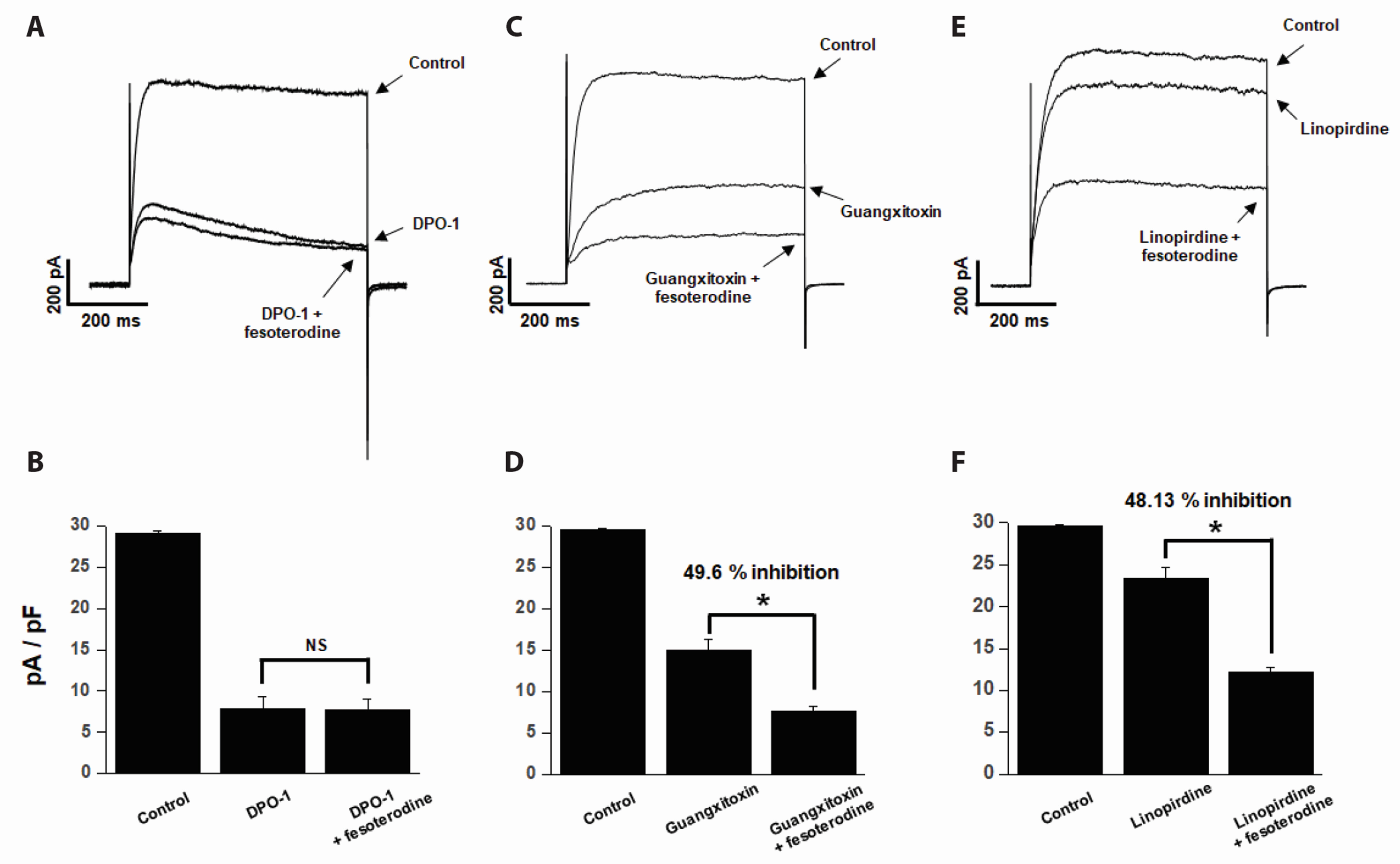
- Fesoterodine, an antimuscarinic drug, is widely used to treat overactive bladder syndrome. However, there is little information about its effects on vascular K+ channels. In this study, voltage-dependent K+ (Kv) channel inhibition by fesoterodine was investigated using the patch-clamp technique in rabbit coronary artery. In whole-cell patches, the addition of fesoterodine to the bath inhibited the Kv currents in a concentration-dependent manner, with an IC50 value of 3.19 ± 0.91 μM and a Hill coefficient of 0.56 ± 0.03. Although the drug did not alter the voltage-dependence of steady-state activation, it shifted the steady-state inactivation curve to a more negative potential, suggesting that fesoterodine affects the voltage-sensor of the Kv channel. Inhibition by fesoterodine was significantly enhanced by repetitive train pulses (1 or 2 Hz). Furthermore, it significantly increased the recovery time constant from inactivation, suggesting that the Kv channel inhibition by fesoterodine is use (state)-dependent. Its inhibitory effect disappeared by pretreatment with a Kv 1.5 inhibitor. However, pretreatment with Kv2.1 or Kv7 inhibitors did not affect the inhibitory effects on Kv channels. Based on these results, we conclude that fesoterodine inhibits vascular Kv channels (mainly the Kv1.5 subtype) in a concentration- and use (state)-dependent manner, independent of muscarinic receptor antagonism.
Keyword
Figure
Reference
-
1. Abrams P, Andersson KE. 2007; Muscarinic receptor antagonists for overactive bladder. BJU Int. 100:987–1006. DOI: 10.1111/j.1464-410X.2007.07205.x. PMID: 17922784.
Article2. Andersson KE, Campeau L, Olshansky B. 2011; Cardiac effects of muscarinic receptor antagonists used for voiding dysfunction. Br J Clin Pharmacol. 72:186–196. DOI: 10.1111/j.1365-2125.2010.03813.x. PMID: 21595741. PMCID: PMC3162648.
Article3. Ellsworth P, Berriman SJ, Brodsky M. 2009; Fesoterodine: a new agent for treating overactive bladder. Am J Manag Care. 15(4 Suppl):S115–S117. PMID: 19355800.4. Vella M, Cardozo L. 2011; Review of fesoterodine. Expert Opin Drug Saf. 10:805–808. DOI: 10.1517/14740338.2011.591377. PMID: 21639817.
Article5. Kuzmin IV, Al-Shukri SK. 2020; Fesoterodine for the treatment of overactive bladder: pharmacological bases and clinical results. Urol Rep. 10:163–171. https://doi.org/10.17816/uroved102163-171. DOI: 10.17816/uroved102163-171.
Article6. Patel N, Wisniowska B, Polak S. 2018; Virtual thorough QT (TQT) trial-extrapolation of in vitro cardiac safety data to in vivo situation using multi-scale physiologically based ventricular cell-wall model exemplified with tolterodine and fesoterodine. AAPS J. 20:83. DOI: 10.1208/s12248-018-0244-3. PMID: 29995258.
Article7. Dogan MF, Yildiz O, Arslan SO, Ulusoy KG. 2019; Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundam Clin Pharmacol. 33:504–523. DOI: 10.1111/fcp.12461. PMID: 30851197.
Article8. Kim HS, Li H, Kim HW, Shin SE, Seo MS, An JR, Ha KS, Han ET, Hong SH, Choi IW, Choi G, Lee DS, Park WS. 2017; Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Korean J Physiol Pharmacol. 21:415–421. DOI: 10.4196/kjpp.2017.21.4.415. PMID: 28706455. PMCID: PMC5507780.
Article9. Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. 2010; Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog Biophys Mol Biol. 103:95–101. DOI: 10.1016/j.pbiomolbio.2009.10.001. PMID: 19835907.
Article10. Nieves-Cintrón M, Syed AU, Nystoriak MA, Navedo MF. 2018; Regulation of voltage-gated potassium channels in vascular smooth muscle during hypertension and metabolic disorders. Microcirculation. 25:e12423. DOI: 10.1111/micc.12423. PMID: 29044853. PMCID: PMC5760350.
Article11. Sobey CG. 2001; Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol. 21:28–38. DOI: 10.1161/01.ATV.21.1.28. PMID: 11145930.
Article12. Park WS, Firth AL, Han J, Ko EA. 2010; Patho-, physiological roles of voltage-dependent K+ channels in pulmonary arterial smooth muscle cells. J Smooth Muscle Res. 46:89–105. DOI: 10.1540/jsmr.46.89. PMID: 20551590.
Article13. Cook NS. 1989; Effect of some potassium channel blockers on contractile responses of the rabbit aorta. J Cardiovasc Pharmacol. 13:299–306. DOI: 10.1097/00005344-198902000-00019. PMID: 2468961.
Article14. Yuan XJ. 1995; Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 77:370–378. DOI: 10.1161/01.RES.77.2.370. PMID: 7542182.
Article15. Bubolz AH, Li H, Wu Q, Liu Y. 2005; Enhanced oxidative stress impairs cAMP-mediated dilation by reducing Kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 289:H1873–H1880. DOI: 10.1152/ajpheart.00357.2005. PMID: 15937095.16. Jackson WF. 2018; KV channels and the regulation of vascular smooth muscle tone. Microcirculation. 25:e12421. DOI: 10.1111/micc.12421. PMID: 28985443. PMCID: PMC5760307.17. Lin ZY, Chen LM, Zhang J, Pan XD, Zhu YG, Ye QY, Huang HP, Chen XC. 2012; Ginsenoside Rb1 selectively inhibits the activity of L-type voltage-gated calcium channels in cultured rat hippocampal neurons. Acta Pharmacol Sin. 33:438–444. DOI: 10.1038/aps.2011.181. PMID: 22407229. PMCID: PMC4003359.
Article18. Wu SN, Hsu MC, Liao YK, Wu FT, Jong YJ, Lo YC. 2012; Evidence for inhibitory effects of flupirtine, a centrally acting analgesic, on delayed rectifier K+ currents in motor neuron-like cells. Evid Based Complement Alternat Med. 2012:148403. DOI: 10.1155/2012/148403. PMID: 22888361. PMCID: PMC3408763.19. Ney P, Pandita RK, Newgreen DT, Breidenbach A, Stöhr T, Andersson KE. 2008; Pharmacological characterization of a novel investigational antimuscarinic drug, fesoterodine, in vitro and in vivo. BJU Int. 101:1036–1042. DOI: 10.1111/j.1464-410X.2007.07358.x. PMID: 18279452.
Article20. Seo MS, An JR, Jung HS, Jung WK, Choi IW, Na SH, Park H, Bae YM, Park WS. 2020; The muscarinic receptor antagonist tolterodine inhibits voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. Eur J Pharmacol. 870:172921. DOI: 10.1016/j.ejphar.2020.172921. PMID: 31935397.21. Li H, Seo MS, An JR, Jung HS, Ha KS, Han ET, Hong SH, Bae YM, Ryu DR, Park WS. 2019; The anticholinergic drug oxybutynin inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Clin Exp Pharmacol Physiol. 46:1030–1036. DOI: 10.1111/1440-1681.13138. PMID: 31330060.
Article22. Seo MS, An JR, Jung HS, Kang M, Heo R, Han ET, Yang SR, Park H, Jung WK, Choi IW, Bae YM, Na SH, Park WS. 2021; Suppression of voltage-gated K+ channels by darifenacin in coronary arterial smooth muscle cells. Eur J Pharmacol. 891:173707. DOI: 10.1016/j.ejphar.2020.173707. PMID: 33137332.23. Smirnov SV, Tammaro P, Hutchings SR, Smith AL. 2003; Role of voltage-gated K+ (Kv) channels in vascular function. Neurophysiology. 35:234–247. https://doi.org/10.1023/B:NEPH.0000008784.83366.9a. DOI: 10.1023/B:NEPH.0000008784.83366.9a.
Article24. Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. 2011; Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011:497841. DOI: 10.1155/2011/497841. PMID: 21403831. PMCID: PMC3042667.
Article25. Morales-Cano D, Moreno L, Barreira B, Pandolfi R, Chamorro V, Jimenez R, Villamor E, Duarte J, Perez-Vizcaino F, Cogolludo A. 2015; Kv7 channels critically determine coronary artery reactivity: left-right differences and down-regulation by hyperglycaemia. Cardiovasc Res. 106:98–108. DOI: 10.1093/cvr/cvv020. PMID: 25616413.
Article26. Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. 2001; Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 89:1030–1037. DOI: 10.1161/hh2301.100817. PMID: 11717160.
Article27. Li Q, Zhang R, Lü CL, Liu Y, Wang Z, Zhu DL. 2006; The role of subtypes of voltage-gated K+ channels in pulmonary vasoconstriction induced by 15-hydroeicosatetraenoic acid. Yao Xue Xue Bao. 41:412–417. Chinese. PMID: 16848316.28. Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA. 2015; G-protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci U S A. 112:6497–6502. DOI: 10.1073/pnas.1418605112. PMID: 25941381. PMCID: PMC4443360.
Article29. Mani BK, Robakowski C, Brueggemann LI, Cribbs LL, Tripathi A, Majetschak M, Byron KL. 2016; Kv7.5 potassium channel subunits are the primary targets for PKA-dependent enhancement of vascular smooth muscle Kv7 currents. Mol Pharmacol. 89:323–334. DOI: 10.1124/mol.115.101758. PMID: 26700561. PMCID: PMC4767407.
Article30. An JR, Li H, Seo MS, Park WS. 2018; Inhibition of voltage-dependent K+ current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone. Korean J Physiol Pharmacol. 22:597–605. DOI: 10.4196/kjpp.2018.22.5.597. PMID: 30181706. PMCID: PMC6115351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent Kâ» channels in coronary arterial smooth muscle cells
- Effects of Tamoxifen on the Voltage-dependent Ionic Currents in Mouse Colonic Smooth Muscle Cells
- Nortriptyline, a tricyclic antidepressant, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells
- Encainide, a class Ic anti-arrhythmic agent, blocks voltage-dependent potassium channels in coronary artery smooth muscle cells