Korean J Physiol Pharmacol.
2022 Sep;26(5):389-396. 10.4196/kjpp.2022.26.5.389.
HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Pusan National University, Yangsan 50612, Korea
- 2Department of Laboratory Medicine, Pusan National University Hospital, Busan 49241, Korea
- 3Department of Anatomy, School of Medicine, Pusan National University, Yangsan 50612, Korea
- KMID: 2532768
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.389
Abstract
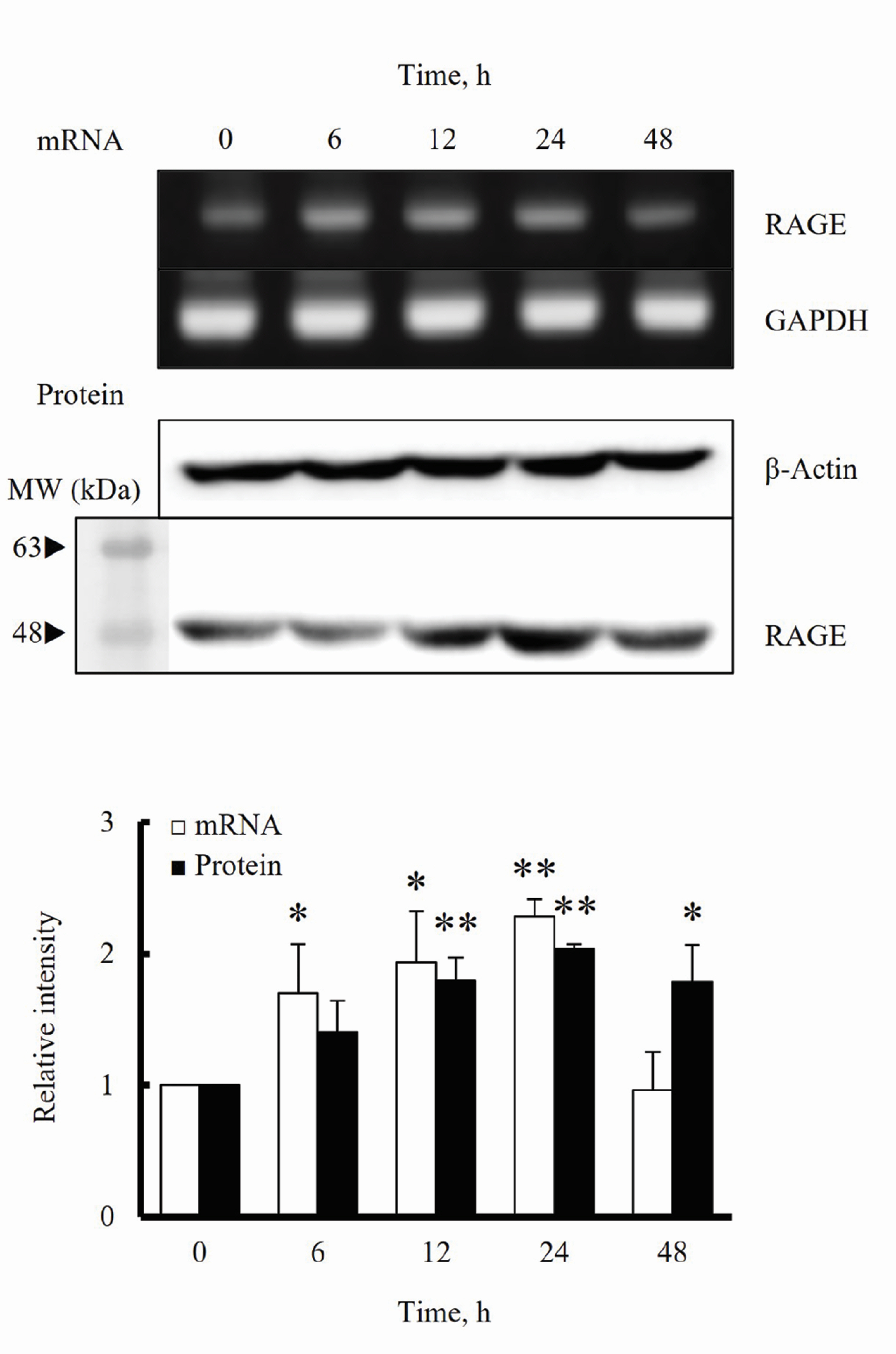
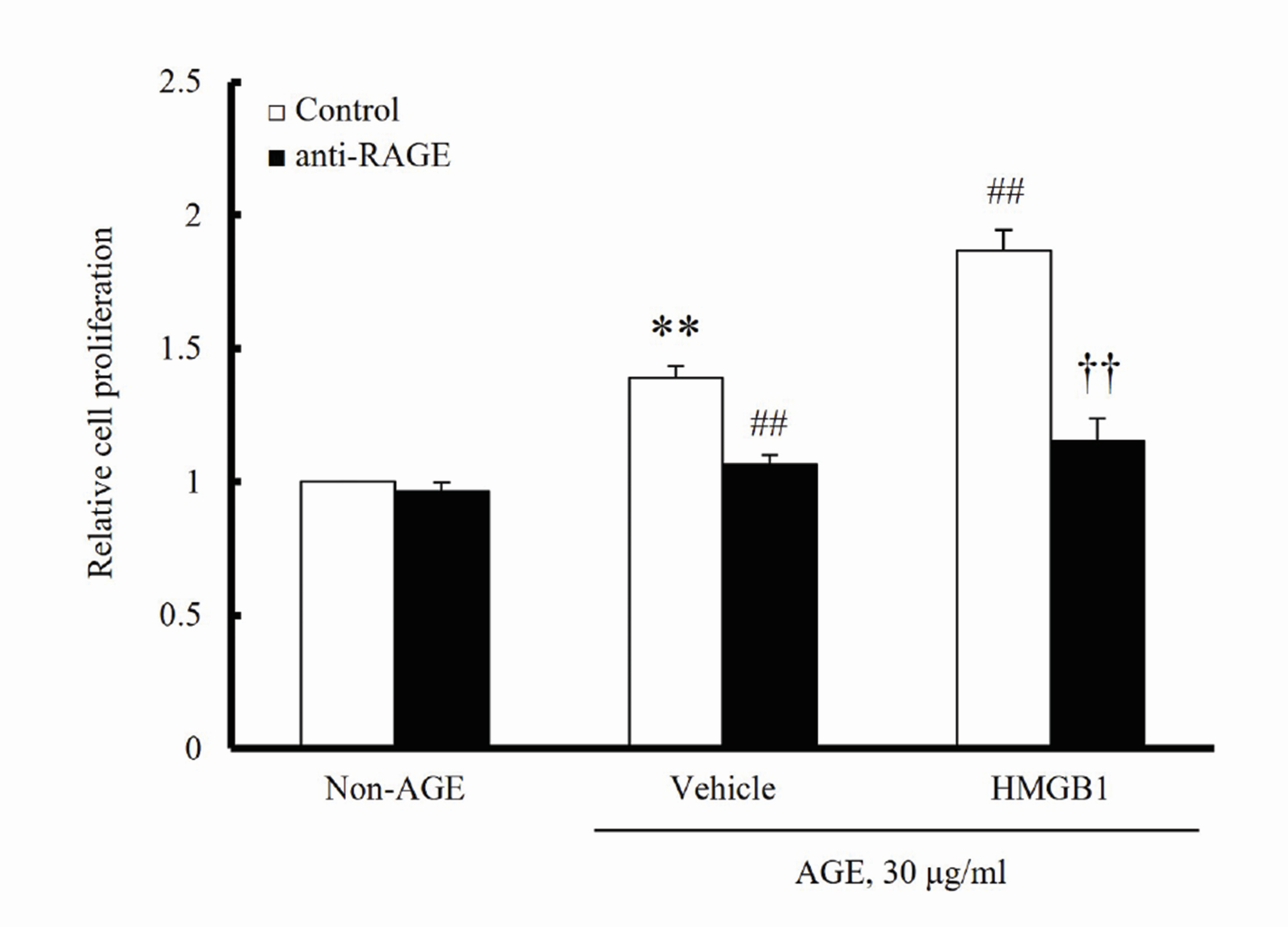
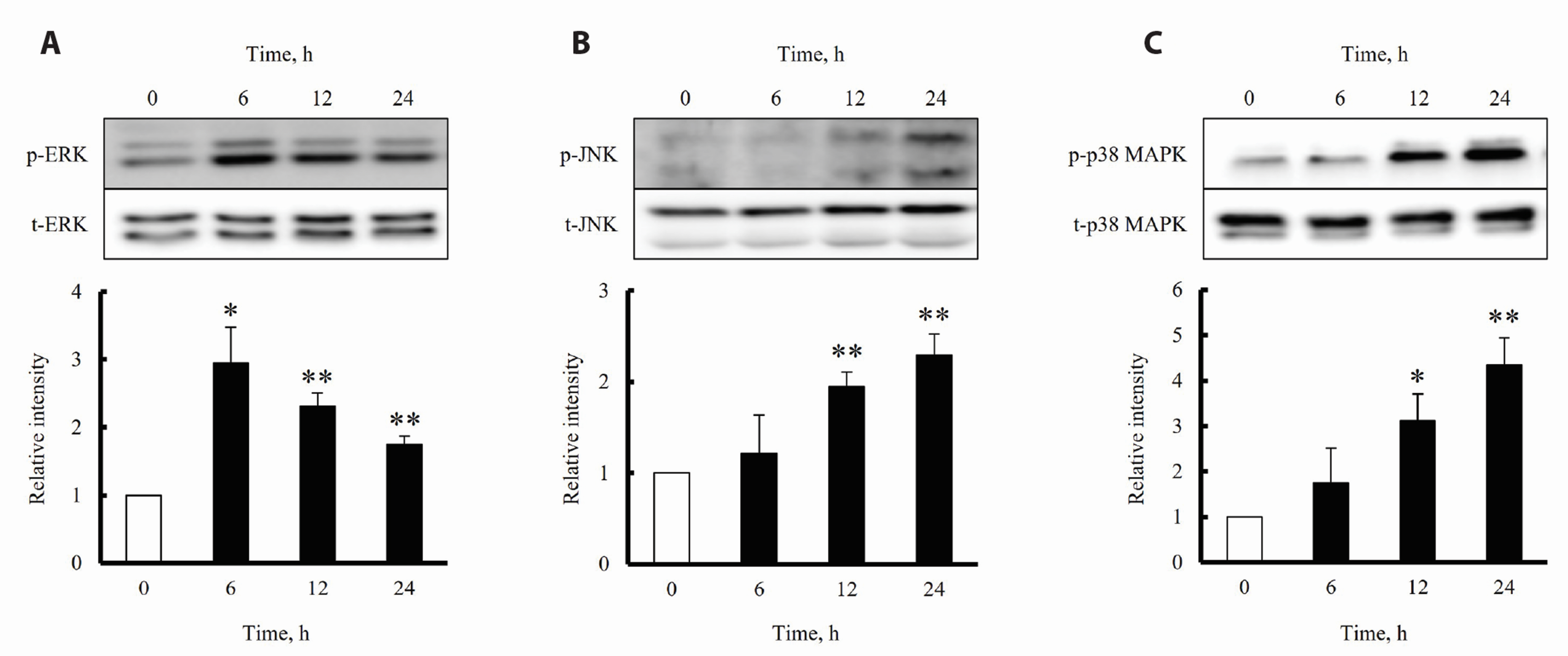
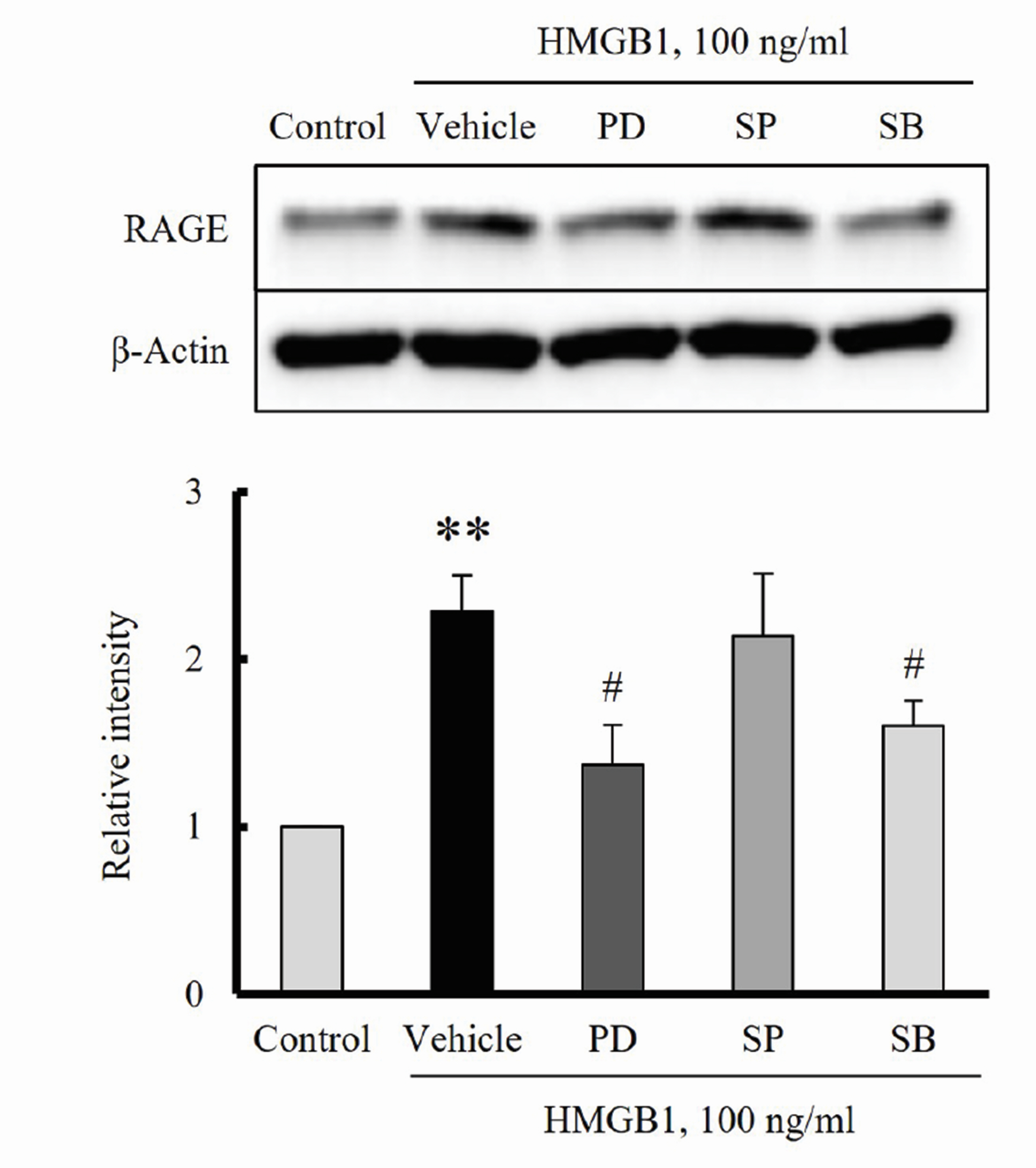
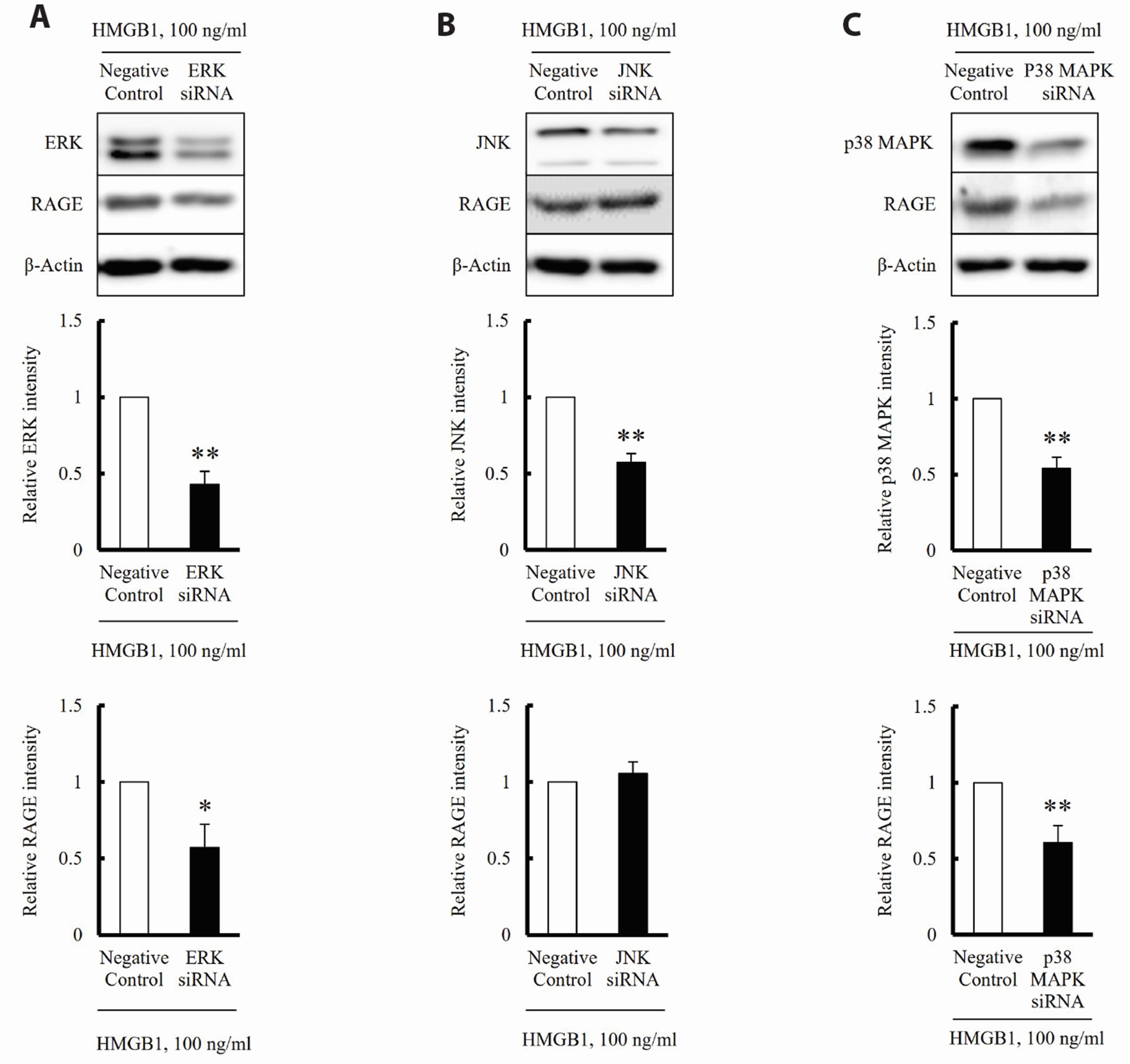
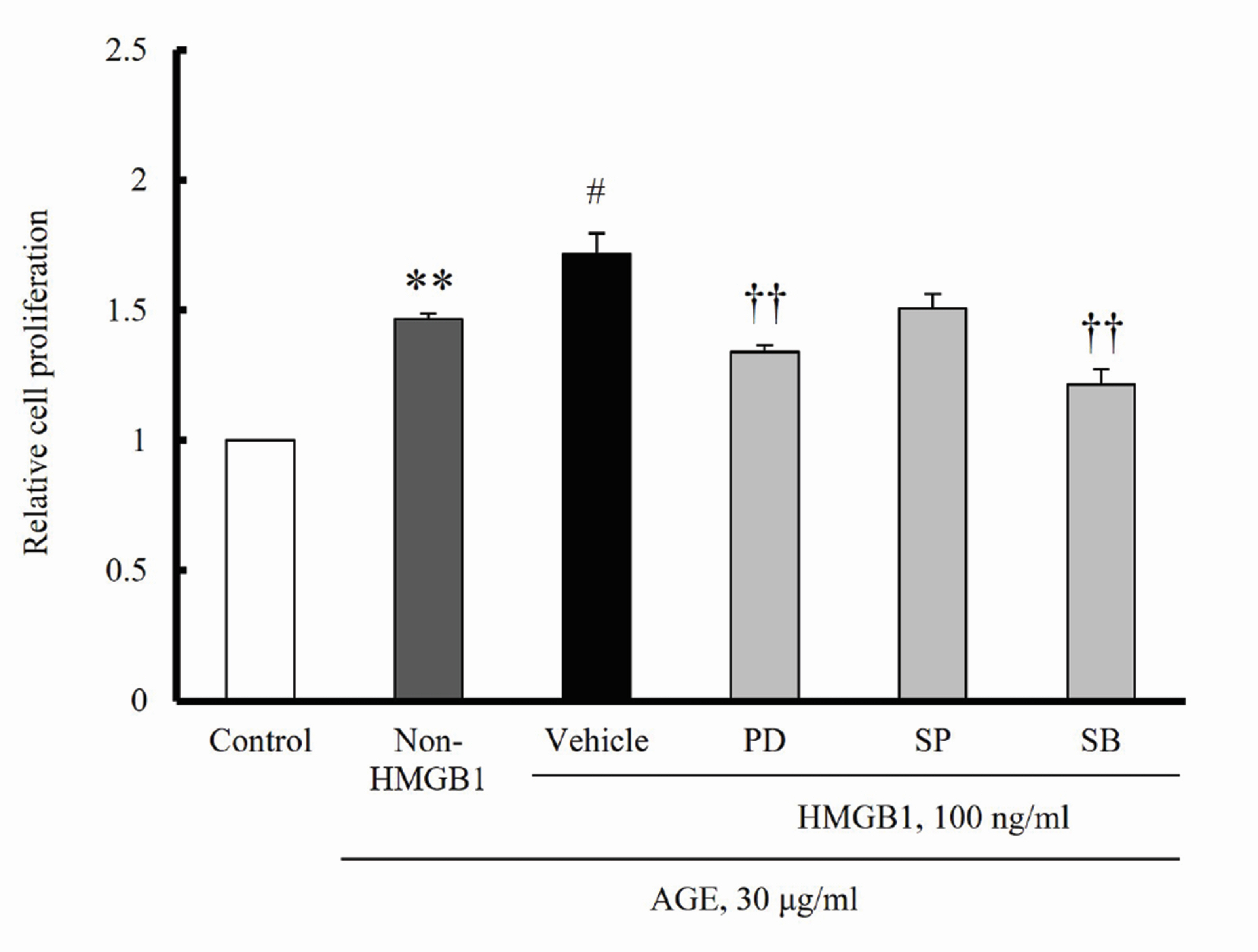
- The increased expression of receptors for advanced glycation endproduct (RAGE) is known as a key player in the progression of vascular remodeling. However, the precise signal pathways regulating RAGE expression in vascular smooth muscle cells (VSMCs) in the injured vasculatures are unclear. Given the importance of mitogen-activated protein kinase (MAPK) signaling in cell proliferation, we investigated the importance of MAPK signaling in high-mobility group box 1 (HMGB1)-induced RAGE expression in VSMCs. In HMGB1 (100 ng/ml)-stimulated human VSMCs, the expression of RAGE mRNA and protein was increased in association with an increase in AGE-induced VSMC proliferation. The HMGB1-induced RAGE expression was attenuated in cells pretreated with inhibitors for ERK (PD98059, 10 μM) and p38 MAPK (SB203580, 10 μM) as well as in cells deficient in ERK and p38 MAPK using siRNAs, but not in cells deficient of JNK signaling. In cells stimulated with HMGB1, the phosphorylation of ERK, JNK, and p38 MAPK was increased. This increase in ERK and p38 MAPK phosphorylation was inhibited by p38 MAPK and ERK inhibitors, respectively, but not by JNK inhibitor. Moreover, AGE-induced VSMC proliferation in HMGB1-stimulated cells was attenuated in cells treated with ERK and p38 MAPK inhibitors. Taken together, our results indicate that ERK and p38 MAPK signaling are involved in RAGE expression in HMGB1-stimulated VSMCs. Thus, the ERK/p38 MAPKRAGE signaling axis in VSMCs was suggested as a potential therapeutic target for vascular remodeling in the injured vasculatures.
Keyword
Figure
Reference
-
1. Land WG. 2013; Chronic allograft dysfunction: a model disorder of innate immunity. Biomed J. 36:209–228. DOI: 10.4103/2319-4170.117622. PMID: 24225188.
Article2. Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. 2009; The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 86:655–662. DOI: 10.1189/jlb.0908548. PMID: 19564572.
Article3. Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. 2004; Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 24:2320–2325. DOI: 10.1161/01.ATV.0000145573.36113.8a. PMID: 15374849.
Article4. Cai Y, Sukhova GK, Wong HK, Xu A, Tergaonkar V, Vanhoutte PM, Tang EH. 2015; Rap1 induces cytokine production in pro-inflammatory macrophages through NFκB signaling and is highly expressed in human atherosclerotic lesions. Cell Cycle. 14:3580–3592. DOI: 10.1080/15384101.2015.1100771. PMID: 26505215. PMCID: PMC4825742.5. Zou H, Yang Y, Gao M, Zhang B, Ming B, Sun Y, Chen H, Tang X, Chen Z, Xiong P, Xu Y, Fang M, Tan Z, Gong F, Zheng F. 2014; HMGB1 is involved in chronic rejection of cardiac allograft via promoting inflammatory-like mDCs. Am J Transplant. 14:1765–1777. DOI: 10.1111/ajt.12781. PMID: 24984831.
Article6. Wang K, Li W, Yu Q, Guo B, Yang B, Zhang C, Li M, Li J, Hu S, Zheng Q, Song Z. 2017; High mobility group box 1 mediates interferon-γ-induced phenotypic modulation of vascular smooth muscle cells. J Cell Biochem. 118:518–529. DOI: 10.1002/jcb.25682. PMID: 27579780.
Article7. Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME. 2006; Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 20:2565–2566. DOI: 10.1096/fj.06-5867fje. PMID: 17060403.
Article8. Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. 2014; The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 33:567–577. DOI: 10.1038/onc.2012.631. PMID: 23318458. PMCID: PMC3795800.
Article9. Andersson U, Tracey KJ. 2011; HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 29:139–162. DOI: 10.1146/annurev-immunol-030409-101323. PMID: 21219181. PMCID: PMC4536551.
Article10. Jang EJ, Baek SE, Kim EJ, Park SY, Kim CD. 2019; HMGB1 enhances AGE-mediated VSMC proliferation via an increase in 5-LO-linked RAGE expression. Vascul Pharmacol. 118-119:106559. DOI: 10.1016/j.vph.2019.04.001. PMID: 30954689.
Article11. Leclerc E, Fritz G, Vetter SW, Heizmann CW. 2009; Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 1793:993–1007. DOI: 10.1016/j.bbamcr.2008.11.016. PMID: 19121341.
Article12. Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. 1996; RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 382:685–691. DOI: 10.1038/382685a0. PMID: 8751438.
Article13. Bierhaus A, Nawroth PP. 2009; Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 52:2251–2263. DOI: 10.1007/s00125-009-1458-9. PMID: 19636529.
Article14. Newby AC. 2006; Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 69:614–624. DOI: 10.1016/j.cardiores.2005.08.002. PMID: 16266693.
Article15. Rakesh K, Agrawal DK. 2005; Cytokines and growth factors involved in apoptosis and proliferation of vascular smooth muscle cells. Int Immunopharmacol. 5:1487–1506. DOI: 10.1016/j.intimp.2005.05.003. PMID: 16023601.
Article16. Doran AC, Meller N, McNamara CA. 2008; Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 28:812–819. DOI: 10.1161/ATVBAHA.107.159327. PMID: 18276911. PMCID: PMC2734458.
Article17. Dinarello CA. 1996; Biologic basis for interleukin-1 in disease. Blood. 87:2095–2147. DOI: 10.1182/blood.V87.6.2095.bloodjournal8762095. PMID: 8630372.
Article18. Schwencke C, Schmeisser A, Walter C, Wachter R, Pannach S, Weck B, Braun-Dullaeus RC, Kasper M, Strasser RH. 2005; Decreased caveolin-1 in atheroma: loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc Res. 68:128–135. DOI: 10.1016/j.cardiores.2005.05.004. PMID: 15950204.
Article19. Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. 2015; Human miR-221/222 in physiological and atherosclerotic vascular remodeling. Biomed Res Int. 2015:354517. DOI: 10.1155/2015/354517. PMID: 26221589. PMCID: PMC4499635.
Article20. Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. 2004; Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 24:1266–1271. DOI: 10.1161/01.ATV.0000131783.74034.97. PMID: 15142859.21. Simard E, Söllradl T, Maltais JS, Boucher J, D'Orléans-Juste P, Grandbois M. 2015; Receptor for advanced glycation end-products signaling interferes with the vascular smooth muscle cell contractile phenotype and function. PLoS One. 10:e0128881. DOI: 10.1371/journal.pone.0128881. PMID: 26248341. PMCID: PMC4527751.
Article22. Schmidt AM, Stern DM. 2000; RAGE: a new target for the prevention and treatment of the vascular and inflammatory complications of diabetes. Trends Endocrinol Metab. 11:368–375. DOI: 10.1016/S1043-2760(00)00311-8. PMID: 11042467.
Article23. Schmidt AM, Yan SD, Yan SF, Stern DM. 2001; The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 108:949–955. DOI: 10.1172/JCI200114002. PMID: 11581294. PMCID: PMC200958.
Article24. Umahara T, Uchihara T, Koyama S, Hashimoto T, Akimoto J, Haraoka J, Iwamoto T. 2014; Local extension of HMGB1 in atherosclerotic lesions of human main cerebral and carotid arteries. Histol Histopathol. 29:235–242. DOI: 10.14670/HH-29.235. PMID: 23929500.25. Indolfi C, Torella D, Cavuto L, Davalli AM, Coppola C, Esposito G, Carriero MV, Rapacciuolo A, Di Lorenzo E, Stabile E, Perrino C, Chieffo A, Pardo F, Chiariello M. 2001; Effects of balloon injury on neointimal hyperplasia in streptozotocin-induced diabetes and in hyperinsulinemic nondiabetic pancreatic islet-transplanted rats. Circulation. 103:2980–2986. DOI: 10.1161/01.CIR.103.24.2980. PMID: 11413090.
Article26. Won SM, Park YH, Kim HJ, Park KM, Lee WJ. 2006; Catechins inhibit angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Exp Mol Med. 38:525–534. DOI: 10.1038/emm.2006.62. PMID: 17079869.
Article27. Liang Y, Hou C, Kong J, Wen H, Zheng X, Wu L, Huang H, Chen Y. 2015; HMGB1 binding to receptor for advanced glycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol Cell Biochem. 405:63–71. DOI: 10.1007/s11010-015-2396-0. PMID: 25862459.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Porphyromonas gingivalis Lipopolysaccharide Regulates Migration of Vascular Smooth Muscle Cells
- Lobaric Acid Inhibits VCAM-1 Expression in TNF-alpha-Stimulated Vascular Smooth Muscle Cells via Modulation of NF-kappaB and MAPK Signaling Pathways
- Mechanisms Involved in the Inhibitory Effects of Mycophenolic Acid on the PDGF-induced Proliferation of Vascular Smooth Muscle Cells
- Roles of ERK and NF-kappa B in Interleukin-8 Expression in Response to Heat Shock Protein 22 in Vascular Smooth Muscle Cells
- The p38 mitogen-activated protein kinase is involved in stress-induced phospholipase D activation in vascular smooth muscle cells