Korean J Physiol Pharmacol.
2022 Sep;26(5):357-365. 10.4196/kjpp.2022.26.5.357.
Mitochondrial energy metabolic transcriptome profiles during cardiac differentiation from mouse and human pluripotent stem cells
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Inje University College of Medicine, Ilsan Paik Hospital, Cardiac & Vascular Center, Goyang 10380, Korea
- 2Cardiovascular and Metabolic Disease Center, Smart Marine Therapeutics Center, Inje University College of Medicine, Busan 47392, Korea
- 3Department of Physiology, Department of Health Sciences and Technology, BK21 Plus Project Team, Inje University College of Medicine, Busan 47392, Korea
- 4Department of Physiology, School of Medicine, Pusan National University, Yangsan 50612, Korea
- 5Research Institute of Convergence Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan 50612, Korea
- KMID: 2532765
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.357
Abstract
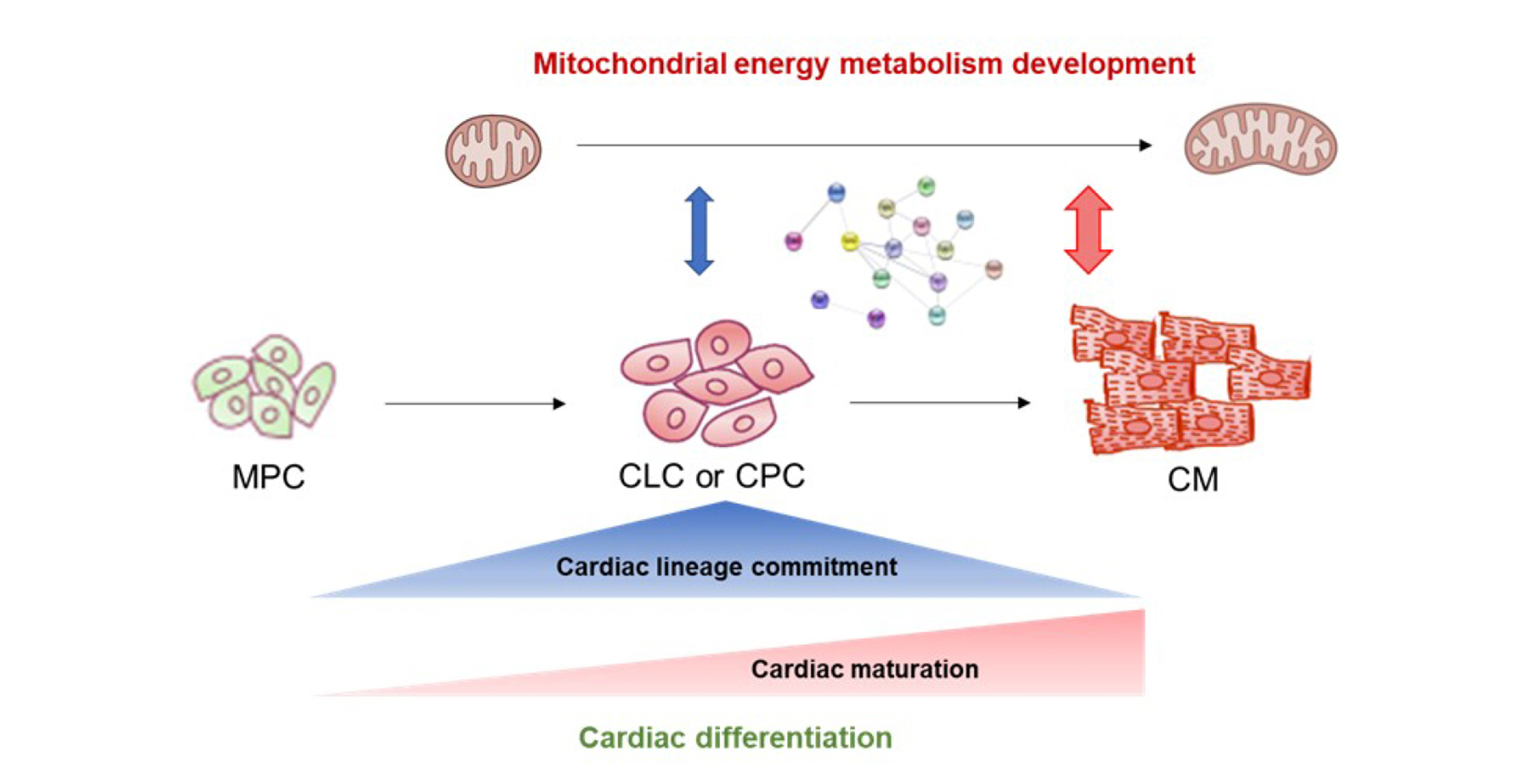
- Simultaneous myofibril and mitochondrial development is crucial for the cardiac differentiation of pluripotent stem cells (PSCs). Specifically, mitochondrial energy metabolism (MEM) development in cardiomyocytes is essential for the beating function. Although previous studies have reported that MEM is correlated with cardiac differentiation, the process and timing of MEM regulation for cardiac differentiation remain poorly understood. Here, we performed transcriptome analysis of cells at specific stages of cardiac differentiation from mouse embryonic stem cells (mESCs) and human induced PSCs (hiPSCs). We selected MEM genes strongly upregulated at cardiac lineage commitment and in a time-dependent manner during cardiac maturation and identified the protein-protein interaction networks. Notably, MEM proteins were found to interact closely with cardiac maturation-related proteins rather than with cardiac lineage commitment-related proteins. Furthermore, MEM proteins were found to primarily interact with cardiac muscle contractile proteins rather than with cardiac transcription factors. We identified several candidate MEM regulatory genes involved in cardiac lineage commitment (Cck, Bdnf, Fabp4, Cebpα, and Cdkn2a in mESC-derived cells, and CCK and NOS3 in hiPSC-derived cells) and cardiac maturation (Ppargc1α, Pgam2, Cox6a2, and Fabp3 in mESC-derived cells, and PGAM2 and SLC25A4 in hiPSC-derived cells). Therefore, our findings show the importance of MEM in cardiac maturation.
Figure
Reference
-
1. Morita Y, Tohyama S. 2020; Metabolic regulation of cardiac differentiation and maturation in pluripotent stem cells: a lesson from heart development. JMA J. 3:193–200. DOI: 10.31662/jmaj.2020-0036. PMID: 33150253. PMCID: PMC7590396.
Article2. Garbern JC, Lee RT. 2021; Mitochondria and metabolic transitions in cardiomyocytes: lessons from development for stem cell-derived cardiomyocytes. Stem Cell Res Ther. 12:177. DOI: 10.1186/s13287-021-02252-6. PMID: 33712058. PMCID: PMC7953594. PMID: 26365eb57d8140289dd56323c91b3762.
Article3. Cho SW, Kim HK, Sung JH, Han J. 2021; Stage specific transcriptome profiles at cardiac lineage commitment during cardiomyocyte differentiation from mouse and human pluripotent stem cells. BMB Rep. 54:464–469. DOI: 10.5483/BMBRep.2021.54.9.046. PMID: 34120677. PMCID: PMC8505231.
Article4. Kamps JA, Krenning G. 2016; Micromanaging cardiac regeneration: targeted delivery of microRNAs for cardiac repair and regeneration. World J Cardiol. 8:163–179. DOI: 10.4330/wjc.v8.i2.163. PMID: 26981212. PMCID: PMC4766267.
Article5. Hong SP, Song S, Cho SW, Lee S, Koh BI, Bae H, Kim KH, Park JS, Do HS, Im I, Heo HJ, Ko TH, Park JH, Youm JB, Kim SJ, Kim I, Han J, Han YM, Koh GY. 2017; Generation of PDGFRα+ cardioblasts from pluripotent stem cells. Sci Rep. 7:41840. DOI: 10.1038/srep41840. PMID: 28165490. PMCID: PMC5292955.
Article6. Hong SP, Song S, Lee S, Jo H, Kim HK, Han J, Park JH, Cho SW. 2019; Regenerative potential of mouse embryonic stem cell-derived PDGFRα+ cardiac lineage committed cells in infarcted myocardium. World J Stem Cells. 11:44–54. DOI: 10.4252/wjsc.v11.i1.44. PMID: 30705714. PMCID: PMC6354102.
Article7. Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. 2011; Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 8:228–240. DOI: 10.1016/j.stem.2010.12.008. PMID: 21295278.
Article8. Takeda M, Kanki Y, Masumoto H, Funakoshi S, Hatani T, Fukushima H, Izumi-Taguchi A, Matsui Y, Shimamura T, Yoshida Y, Yamashita JK. 2018; Identification of cardiomyocyte-fated progenitors from human-induced pluripotent stem cells marked with CD82. Cell Rep. 22:546–556. DOI: 10.1016/j.celrep.2017.12.057. PMID: 29320747.
Article9. Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. 2013; Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 8:162–175. DOI: 10.1038/nprot.2012.150. PMID: 23257984. PMCID: PMC3612968.
Article10. Porter GA Jr, Hom J, Hoffman D, Quintanilla R, de Mesy Bentley K, Sheu SS. 2011; Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog Pediatr Cardiol. 31:75–81. DOI: 10.1016/j.ppedcard.2011.02.002. PMID: 21603067. PMCID: PMC3096664.
Article11. Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, et al. 2013; Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 12:127–137. DOI: 10.1016/j.stem.2012.09.013. PMID: 23168164.
Article12. Heo HJ, Kim HK, Youm JB, Cho SW, Song IS, Lee SY, Ko TH, Kim N, Ko KS, Rhee BD, Han J. 2016; Mitochondrial pyruvate dehydrogenase phosphatase 1 regulates the early differentiation of cardiomyocytes from mouse embryonic stem cells. Exp Mol Med. 48:e254. DOI: 10.1038/emm.2016.70. PMID: 27538372. PMCID: PMC5007642.
Article13. Cho SW, Park JS, Heo HJ, Park SW, Song S, Kim I, Han YM, Yamashita JK, Youm JB, Han J, Koh GY. 2014; Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells. J Am Heart Assoc. 3:e000693. DOI: 10.1161/JAHA.113.000693. PMID: 24627421. PMCID: PMC4187507.
Article14. Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, Ritterhoff J, Zhao L, Kolwicz SC Jr, Pabon L, Reinecke H, Sniadecki NJ, Tian R, Ruohola-Baker H, Xu H, Murry CE. 2019; Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports. 13:657–668. DOI: 10.1016/j.stemcr.2019.08.013. PMID: 31564645. PMCID: PMC6829750.
Article15. Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hörmann L, Ulmer B, Zhang H, Briganti F, Schweizer M, Hegyi B, Liao Z, Pölönen RP, Ginsburg KS, Lam CK, Serrano R, Wahlquist C, Kreymerman A, Vu M, Amatya PL, Behrens CS, et al. 2020; Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 32:107925. DOI: 10.1016/j.celrep.2020.107925. PMID: 32697997. PMCID: PMC7437654.
Article16. Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, et al. 2017; Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 114:E8372–E8381. DOI: 10.1073/pnas.1707316114. PMID: 28916735. PMCID: PMC5635889.
Article17. Lay JM, Gillespie PJ, Samuelson LC. 1999; Murine prenatal expression of cholecystokinin in neural crest, enteric neurons, and enteroendocrine cells. Dev Dyn. 216:190–200. DOI: 10.1002/(SICI)1097-0177(199910)216:2<190::AID-DVDY9>3.0.CO;2-K. PMID: 10536058.
Article18. Goetze JP, Johnsen AH, Kistorp C, Gustafsson F, Johnbeck CB, Rehfeld JF. 2015; Cardiomyocyte expression and cell-specific processing of procholecystokinin. J Biol Chem. 290:6837–6843. DOI: 10.1074/jbc.M114.622670. PMID: 25627687. PMCID: PMC4358109.
Article19. Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, Bassel-Duby R, Olson EN. 2012; C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 338:1599–1603. DOI: 10.1126/science.1229765. PMID: 23160954. PMCID: PMC3613149.
Article20. Xu R, inivasan SP Sr, Sureshkumar P, Nembo EN, Schäfer C, Semmler J, Matzkies M, Albrechtsen M, Hescheler J, Nguemo F. 2015; Effects of synthetic neural adhesion molecule mimetic peptides and related proteins on the cardiomyogenic differentiation of mouse embryonic stem cells. Cell Physiol Biochem. 35:2437–2450. DOI: 10.1159/000374044. PMID: 25967873.
Article21. Liu Y, Feng Q. 2012; NOing the heart: role of nitric oxide synthase-3 in heart development. Differentiation. 84:54–61. DOI: 10.1016/j.diff.2012.04.004. PMID: 22579300.
Article22. Wang X, Zhou L, Jin J, Yang Y, Song G, Shen Y, Liu H, Liu M, Shi C, Qian L. 2013; Knockdown of FABP3 impairs cardiac development in Zebrafish through the retinoic acid signaling pathway. Int J Mol Sci. 14:13826–13841. DOI: 10.3390/ijms140713826. PMID: 23823803. PMCID: PMC3742220.
Article23. Murphy SA, Miyamoto M, Kervadec A, Kannan S, Tampakakis E, Kambhampati S, Lin BL, Paek S, Andersen P, Lee DI, Zhu R, An SS, Kass DA, Uosaki H, Colas AR, Kwon C. 2021; PGC1/PPAR drive cardiomyocyte maturation at single cell level via YAP1 and SF3B2. Nat Commun. 12:1648. DOI: 10.1038/s41467-021-21957-z. PMID: 33712605. PMCID: PMC7955035. PMID: e529861439104233888f042c1cc8bff3.
Article24. Liu Y, Bai H, Guo F, Thai PN, Luo X, Zhang P, Yang C, Feng X, Zhu D, Guo J, Liang P, Xu Z, Yang H, Lu X. 2020; PGC-1α activator ZLN005 promotes maturation of cardiomyocytes derived from human embryonic stem cells. Aging (Albany NY). 12:7411–7430. DOI: 10.18632/aging.103088. PMID: 32343674. PMCID: PMC7202542.
Article25. Narula N, Zaragoza MV, Sengupta PP, Li P, Haider N, Verjans J, Waymire K, Vannan M, Wallace DC. 2011; Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis. JACC Cardiovasc Imaging. 4:1–10. DOI: 10.1016/j.jcmg.2010.06.018. PMID: 21232697. PMCID: PMC4023693.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?
- Mitochondrial pyruvate dehydrogenase phosphatase 1 regulates the early differentiation of cardiomyocytes from mouse embryonic stem cells
- New Advances in Human X Chromosome Status from a Developmental and Stem Cell Biology
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Neuronal Differentiation of a Human Induced Pluripotent Stem Cell Line (FS-1) Derived from Newborn Foreskin Fibroblasts