J Korean Med Sci.
2022 Aug;37(31):e244. 10.3346/jkms.2022.37.e244.
IntraBrain Injector (IBI): A StereotacticGuided Device for Repeated Delivery of Therapeutic Agents Into the Brain Parenchyma
- Affiliations
-
- 1Cell and Gene Therapy Institute, Samsung Medical Center, Seoul, Korea
- 2ANYMEDI Inc., Seoul, Korea
- 3Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Seohan Care Co., Ltd., Gimpo, Korea
- 6Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Neuroscience Center, Samsung Medical Center, Seoul, Korea
- 8Samsung Alzheimer Convergence Research Center, Samsung Medical Center, Seoul, Korea
- 9Department of Neurology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 10Sungkyunkwan University School of Medicine, Seoul, Korea
- 11Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 12Biomedical Research Institute, MEDIPOST Co., Ltd., Seongnam, Korea
- KMID: 2532319
- DOI: http://doi.org/10.3346/jkms.2022.37.e244
Abstract
- Background
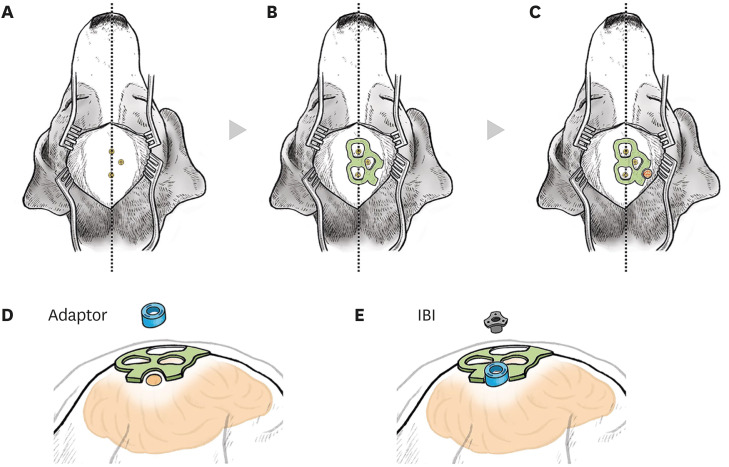
To deliver therapeutics into the brain, it is imperative to overcome the issue of the blood-brain-barrier (BBB). One of the ways to circumvent the BBB is to administer therapeutics directly into the brain parenchyma. To enhance the treatment efficacy for chronic neurodegenerative disorders, repeated administration to the target location is required. However, this increases the number of operations that must be performed. In this study, we developed the IntraBrain Injector (IBI), a new implantable device to repeatedly deliver therapeutics into the brain parenchyma.
Methods
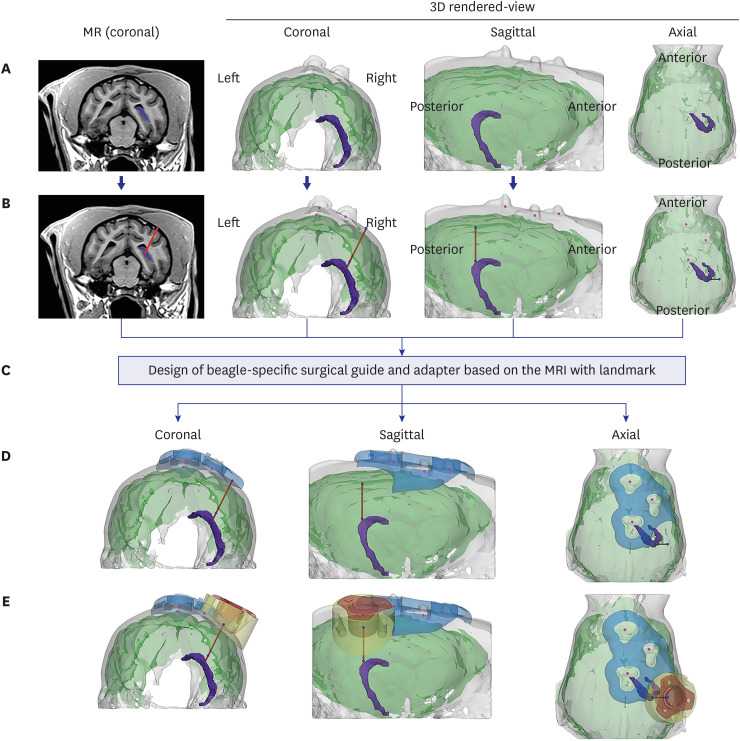
We designed and fabricated IBI with medical grade materials, and evaluated the efficacy and safety of IBI in 9 beagles. The trajectory of IBI to the hippocampus was simulated prior to surgery and the device was implanted using 3D-printed adaptor and surgical guides. Ferumoxytol-labeled mesenchymal stem cells (MSCs) were injected into the hippocampus via IBI, and magnetic resonance images were taken before and after the administration to analyze the accuracy of repeated injection.
Results
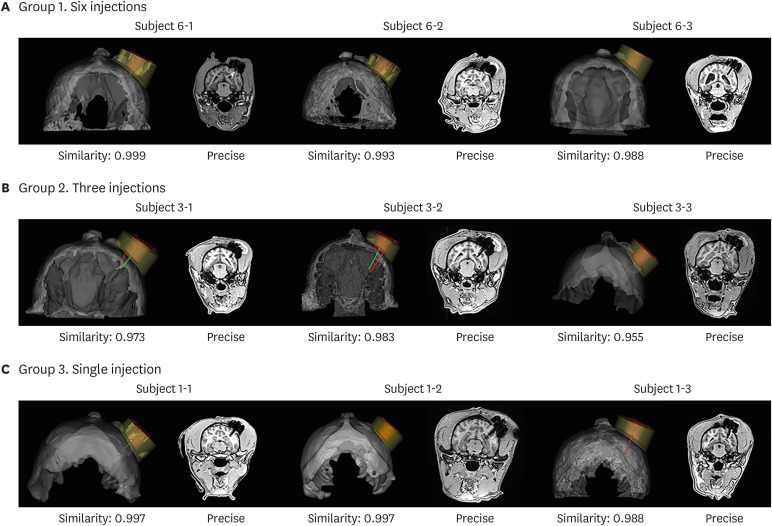
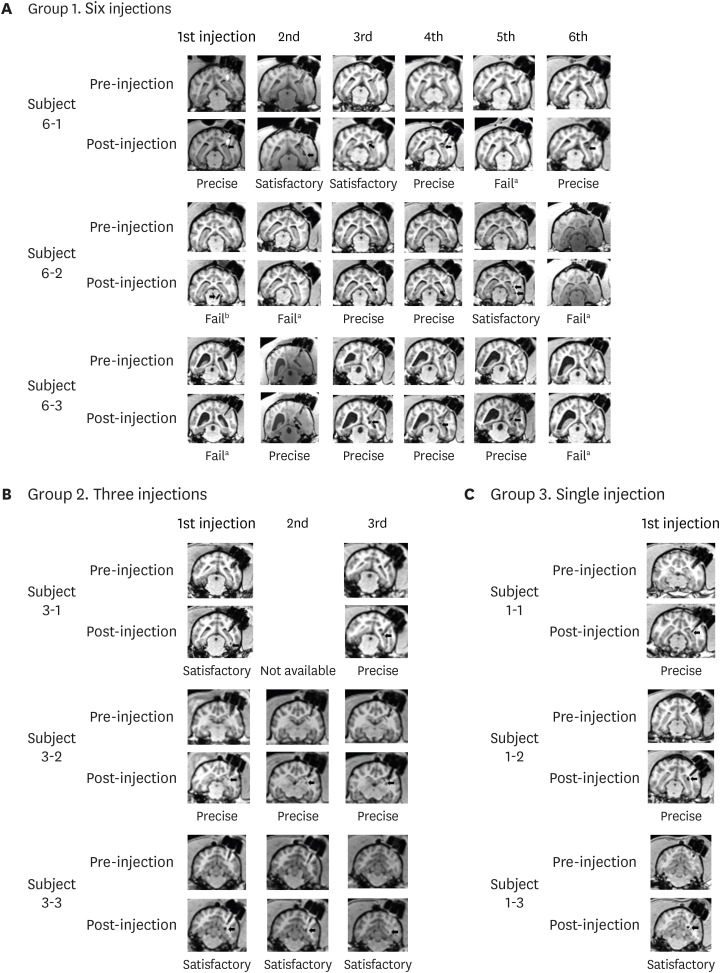
We compared the planned vs. insertion trajectory of IBI to the hippocampus. With a similarity of 0.990 ± 0.001 (mean ± standard deviation), precise targeting of IBI was confirmed by comparing planned vs. insertion trajectories of IBI. Multiple administrations of ferumoxytol-labeled MSCs into the hippocampus using IBI were both feasible and successful (success rate of 76.7%). Safety of initial IBI implantation, repeated administration of therapeutics, and long-term implantation have all been evaluated in this study.
Conclusion
Precise and repeated delivery of therapeutics into the brain parenchyma can be done without performing additional surgeries via IBI implantation.
Keyword
Figure
Reference
-
1. Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019; 15(10):565–581. PMID: 31501588.
Article2. Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003; 348(14):1356–1364. PMID: 12672864.
Article3. Scannevin RH. Therapeutic strategies for targeting neurodegenerative protein misfolding disorders. Curr Opin Chem Biol. 2018; 44:66–74. PMID: 29902695.
Article4. Ahmadian-Moghadam H, Sadat-Shirazi MS, Zarrindast MR. Therapeutic potential of stem cells for treatment of neurodegenerative diseases. Biotechnol Lett. 2020; 42(7):1073–1101. PMID: 32342435.
Article5. Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016; 15(4):275–292. PMID: 26794270.
Article6. Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020; 165-166:1–14. PMID: 31790711.
Article7. Nowak M, Helgeson ME, Mitragotri S. Delivery of nanoparticles and macromolecules across the blood–brain barrier. Adv Ther. 2020; 3(1):1900073.
Article8. Bors L, Erdő F. Overcoming the blood–brain barrier. challenges and tricks for CNS drug delivery. Sci Pharm. 2019; 87(1):6.
Article9. Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014; 13(9):655–672. PMID: 25103255.
Article10. Barchet TM, Amiji MM. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin Drug Deliv. 2009; 6(3):211–225. PMID: 19290842.
Article11. Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010; 5(6):933–946. PMID: 21082892.
Article12. Drela K, Siedlecka P, Sarnowska A, Domanska-Janik K. Human mesenchymal stem cells in the treatment of neurological diseases. Acta Neurobiol Exp (Warsz). 2013; 73(1):38–56. PMID: 23595282.13. Volkman R, Offen D. Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells. 2017; 35(8):1867–1880. PMID: 28589621.
Article14. Park SE, Lee NK, Lee J, Hwang JW, Choi SJ, Hwang H, et al. Distribution of human umbilical cord blood-derived mesenchymal stem cells in the Alzheimer’s disease transgenic mouse after a single intravenous injection. Neuroreport. 2016; 27(4):235–241. PMID: 26752148.
Article15. Lee NK, Yang J, Chang EH, Park SE, Lee J, Choi SJ, et al. Intra-arterially delivered mesenchymal stem cells are not detected in the brain parenchyma in an Alzheimer’s disease mouse model. PLoS One. 2016; 11(5):e0155912. PMID: 27203695.
Article16. Potts MB, Silvestrini MT, Lim DA. Devices for cell transplantation into the central nervous system: design considerations and emerging technologies. Surg Neurol Int. 2013; 4(Suppl 1):S22–S30. PMID: 23653887.
Article17. Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010; 40(5):385–403. PMID: 20497904.
Article18. Slavc I, Cohen-Pfeffer JL, Gururangan S, Krauser J, Lim DA, Maldaun M, et al. Best practices for the use of intracerebroventricular drug delivery devices. Mol Genet Metab. 2018; 124(3):184–188. PMID: 29793829.
Article19. Kim HJ, Seo SW, Chang JW, Lee JI, Kim CH, Chin J, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimers Dement (N Y). 2015; 1(2):95–102. PMID: 29854930.
Article20. Amer MH, Rose FR, Shakesheff KM, Modo M, White LJ. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. NPJ Regen Med. 2017; 2(1):23. PMID: 29302358.
Article21. Lee NK, Kim HS, Yoo D, Hwang JW, Choi SJ, Oh W, et al. Magnetic resonance imaging of ferumoxytol-labeled human mesenchymal stem cells in the mouse brain. Stem Cell Rev Rep. 2017; 13(1):127–138. PMID: 27757917.
Article22. Bryant LH Jr, Kim SJ, Hobson M, Milo B, Kovacs ZI, Jikaria N, et al. Physicochemical characterization of ferumoxytol, heparin and protamine nanocomplexes for improved magnetic labeling of stem cells. Nanomedicine. 2017; 13(2):503–513. PMID: 27520728.
Article23. Mendez I, Hong M, Smith S, Dagher A, Desrosiers J. Neural transplantation cannula and microinjector system: experimental and clinical experience. Technical note. J Neurosurg. 2000; 92(3):493–499. PMID: 10701543.
Article24. Cunningham MG, Bolay H, Scouten CW, Moore C, Jacoby D, Moskowitz M, et al. Preclinical evaluation of a novel intracerebral microinjection instrument permitting electrophysiologically guided delivery of therapeutics. Neurosurgery. 2004; 54(6):1497–1507. PMID: 15157308.
Article25. Bjarkam CR, Glud AN, Margolin L, Reinhart K, Franklin R, Deding D, et al. Safety and function of a new clinical intracerebral microinjection instrument for stem cells and therapeutics examined in the Göttingen minipig. Stereotact Funct Neurosurg. 2010; 88(1):56–63. PMID: 20051711.
Article26. Silvestrini MT, Yin D, Coppes VG, Mann P, Martin AJ, Larson PS, et al. Radially branched deployment for more efficient cell transplantation at the scale of the human brain. Stereotact Funct Neurosurg. 2013; 91(2):92–103. PMID: 23343609.
Article27. Dagdeviren C, Ramadi KB, Joe P, Spencer K, Schwerdt HN, Shimazu H, et al. Miniaturized neural system for chronic, local intracerebral drug delivery. Sci Transl Med. 2018; 10(425):eaan2742. PMID: 29367347.
Article28. Dunkel P, Chai CL, Sperlágh B, Huleatt PB, Mátyus P. Clinical utility of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2012; 21(9):1267–1308.
Article29. Hussain R, Zubair H, Pursell S, Shahab M. Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018; 8(9):177.
Article30. Mehta AM, Sonabend AM, Bruce JN. Convection-enhanced delivery. Neurotherapeutics. 2017; 14(2):358–371. PMID: 28299724.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biological effects of blood–brain barrier disruption using a focused ultrasound
- Erratum: Correction of the Funding Statement: Feasibility and Therapeutic Effects of a Novel Magnet-Based Device for Hand Rehabilitation: a Pilot Study
- Prevention of infection from contrast agents
- Effects of a Novel Push-through Technique Using the Implantable Collamer Lens Injector System for Graft Delivery during Endothelial Keratoplasty
- Stereotactic Radiosurgery for Metastatic Brain Tumor