Brain Tumor Res Treat.
2022 Jul;10(3):183-189 . 10.14791/btrt.2022.0013.
Leptomeningeal Spread at the Diagnosis of Glioblastoma Multiforme: A Case Report and Literature Review
- Affiliations
-
- 1Department of Neurosurgery, The Armed Forces Capital Hospital, Seongnam, Korea
- KMID: 2532250
- DOI: http://doi.org/10.14791/btrt.2022.0013
Abstract
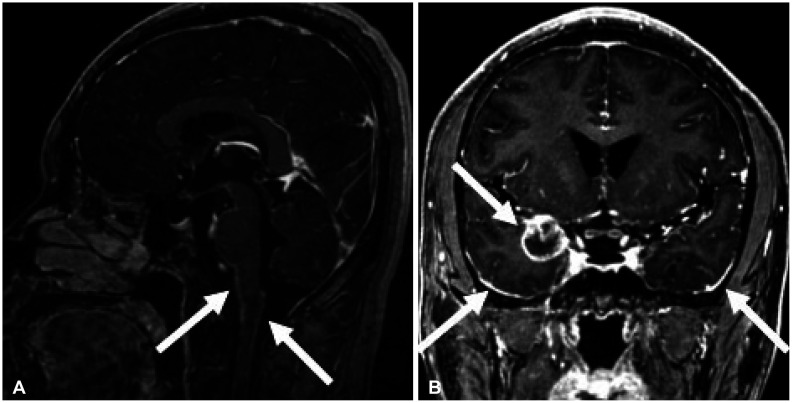
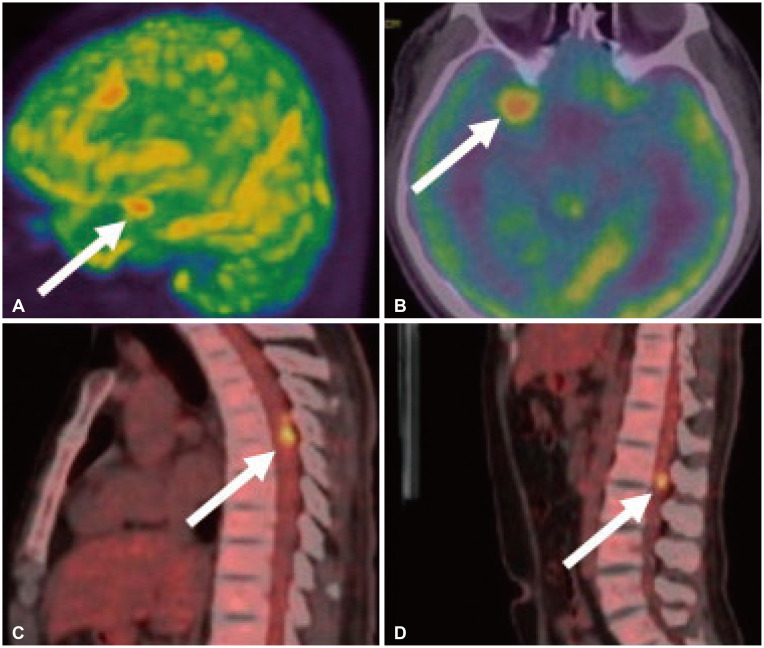
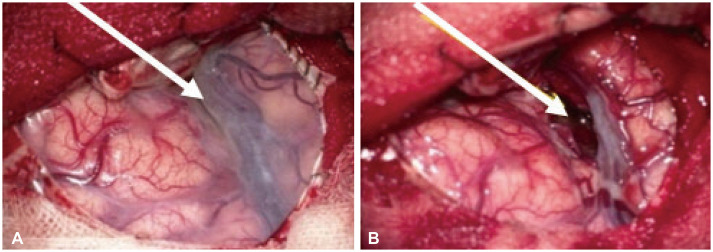
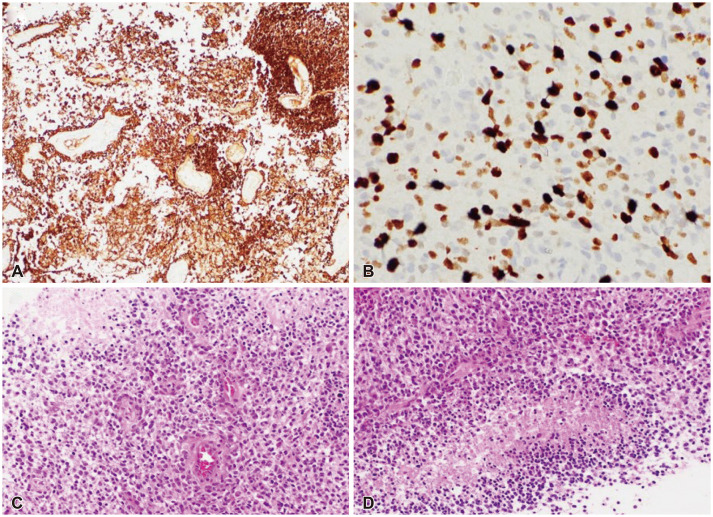
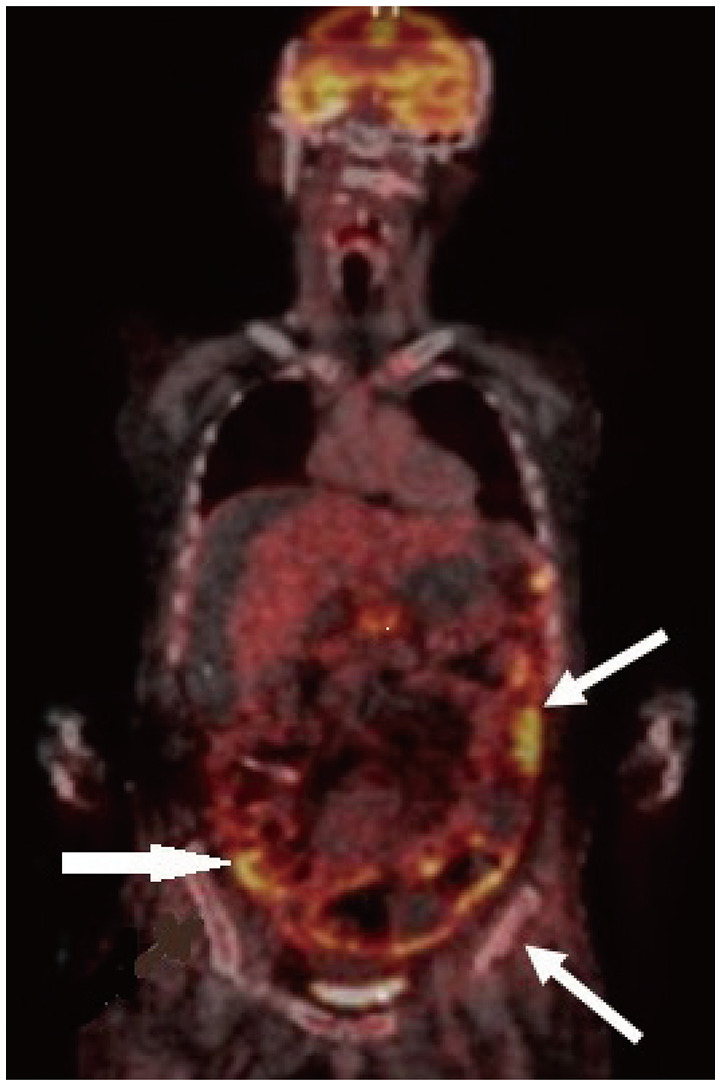
- Approximately two-thirds of glioblastoma (GBM) patients progress to leptomeningeal spread (LMS) within two years. While 90% of LMS cases are diagnosed during the progression and/or recurrence of GBM (defined as secondary LMS), LMS presentation at the time of GBM diagnosis (defined as primary LMS) is very rare. 18 F-fluorodeoxy glucose positron emission tomography computed tomography ( 18F-FDG PET/CT) study helps to diagnose the multifocal spread of the malignant primary brain tumor. Our patient was a 31-year-old man with a tumorous lesion located in the right temporal lobe, a wide area of the leptomeninges, and spinal cord (thoracic 5/6, and lumbar 1 level) involvement as a concurrent manifestation. After the removal of the right temporal tumor, the clinical status progressed rapidly, showing signs of increased intracranial pressure and hydrocephalus caused by LMS. He underwent a ventriculoperitoneal shunt a week after craniotomy. During management, progression of cord compression, paraplegia, bone marrow suppression related to radiochemotherapy, intercurrent infections, and persistent ascites due to peritoneal metastasis of the LMS through the shunt system was observed. The patient finally succumbed to the disease nine months after the diagnosis of simultaneous GBM and LMS. The overall survival of primary LMS with GBM in our case was nine months, which is shorter than that of secondary LMS with GBM. The survival period after the diagnosis of LMS did not seem to be significantly different between primary and secondary LMS. To determine the prognostic effect and difference between primary and secondary LMS, further cooperative studies with large-volume data analysis are warranted.
Keyword
Figure
Reference
-
1. Birzu C, Tran S, Bielle F, Touat M, Mokhtari K, Younan N, et al. Leptomeningeal spread in glioblastoma: diagnostic and therapeutic challenges. Oncologist. 2020; 25:e1763–e1776. PMID: 33394574.2. Autran D, Barrie M, Matta M, Monserrat C, Campello C, Petrirena G, et al. Leptomeningeal gliomatosis: a single institution study of 31 patients. Anticancer Res. 2019; 39:1035–1041. PMID: 30711992.3. Noh JH, Lee MH, Kim WS, Lim DH, Kim ST, Kong DS, et al. Optimal treatment of leptomeningeal spread in glioblastoma: analysis of risk factors and outcome. Acta Neurochir (Wien). 2015; 157:569–576. PMID: 25663100.4. Mandel JJ, Yust-Katz S, Cachia D, Wu J, Liu D, de Groot JF, et al. Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 2014; 120:597–605. PMID: 25168214.5. Zhao KH, Zhang C, Bai Y, Li Y, Kang X, Chen JX, et al. Antiglioma effects of cytarabine on leptomeningeal metastasis of high-grade glioma by targeting the PI3K/Akt/mTOR pathway. Drug Des Devel Ther. 2017; 11:1905–1915.6. Brown MT, Coleman RE, Friedman AH, Friedman HS, McLendon RE, Reiman R, et al. Intrathecal 131I-labeled antitenascin monoclonal antibody 81C6 treatment of patients with leptomeningeal neoplasms or primary brain tumor resection cavities with subarachnoid communication: phase I trial results. Clin Cancer Res. 1996; 2:963–972. PMID: 9816257.7. Chamberlain MC. Combined-modality treatment of leptomeningeal gliomatosis. Neurosurgery. 2003; 52:324–329. discussion 330. PMID: 12535360.8. Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999; 5:3394–3402. PMID: 10589750.9. Leaver KE, Zhang N, Ziskin JL, Vogel H, Recht L, Thomas RP. Response of metastatic glioma to vemurafenib. Neurooncol Pract. 2016; 3:268–271. PMID: 31386052.10. Burger MC, Zeiner PS, Jahnke K, Wagner M, Mittelbronn M, Steinbach JP. Addition of anti-angiogenetic therapy with bevacizumab to chemo- and radiotherapy for leptomeningeal metastases in primary brain tumors. PLoS One. 2016; 11:e0155315. PMID: 27253224.11. Kanemaru Y, Natsumeda M, Okada M, Saito R, Kobayashi D, Eda T, et al. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol Commun. 2019; 7:119. PMID: 31345255.12. Woo PYM, Lam TC, Pu JKS, Li LF, Leung RCY, Ho JMK, et al. Regression of BRAFV600E mutant adult glioblastoma after primary combined BRAF-MEK inhibitor targeted therapy: a report of two cases. Oncotarget. 2019; 10:3818–3826. PMID: 31217909.13. Andersen BM, Miranda C, Hatzoglou V, DeAngelis LM, Miller AM. Leptomeningeal metastases in glioma: The Memorial Sloan Kettering Cancer Center experience. Neurology. 2019; 92:e2483–e2491. PMID: 31019097.14. Kwon JE, Hwang K, Go KO, Wee CW, Kim IA, Kim YJ, et al. Clinical characteristics of high-grade glioma with primary leptomeningeal seeding at initial diagnosis in a single center study. Brain Tumor Res Treat. 2020; 8:77–82. PMID: 33118340.15. Nadkarni T, Hamilton K, Niazi F, Ward M, Okakpu U, Castellani RJ, et al. Histone-mutant glioma presenting as diffuse leptomeningeal disease. CNS Oncol. 2021; 10:CNS75. PMID: 34469205.16. Yamasaki K, Yokogami K, Ohta H, Yamashita S, Uehara H, Sato Y, et al. A case of primary diffuse leptomeningeal gliomatosis. Brain Tumor Pathol. 2014; 31:177–181. PMID: 24473978.17. Dardis C, Milton K, Ashby L, Shapiro W. Leptomeningeal metastases in high-grade adult glioma: development, diagnosis, management, and outcomes in a series of 34 patients. Front Neurol. 2014; 5:220. PMID: 25404928.18. Intriago B, Danús M, Añaños M, Trampal C, Montero M, Calvo N. 18F-FDG PET detection of spinal leptomeningeal metastases from cerebral glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2011; 38:1392. PMID: 21394504.19. Kanai R, Tasaka M, Sejima H, Uchida N, Nakano A, Akiyama Y, et al. Brain stem glioblastoma with multiple large cyst formation and leptomeningeal dissemination in a 4-year-old girl. Brain Dev. 2005; 27:58–61. PMID: 15626543.20. Pohar S, Taylor W, Chandan VS, Shah H, Sagerman RH. Primary presentation of glioblastoma multiforme with leptomeningeal metastasis in the absence of previous craniotomy: a case report. Am J Clin Oncol. 2004; 27:640–641. PMID: 15577447.21. Witham TF, Fukui MB, Meltzer CC, Burns R, Kondziolka D, Bozik ME. Survival of patients with high grade glioma treated with intrathecal thiotriethylenephosphoramide for ependymal or leptomeningeal gliomatosis. Cancer. 1999; 86:1347–1353. PMID: 10506724.22. Reifenberger G, Boström J, Bettag M, Bock WJ, Wechsler W, Kepes JJ. Primary glioblastoma multiforme of the oculomotor nerve. Case report. J Neurosurg. 1996; 84:1062–1066. PMID: 8847574.23. Shuangshoti S, Shuangshoti S. Primary diffuse leptomeningeal glioblastoma multiforme of brainstem and spinal cord clinically mimicking meningitis: case report and review of literature. J Med Assoc Thai. 1996; 79:403–408. PMID: 8855617.24. Giordana MT, Bradac GB, Pagni CA, Marino S, Attanasio A. Primary diffuse leptomeningeal gliomatosis with anaplastic features. Acta Neurochir (Wien). 1995; 132:154–159. PMID: 7754854.25. Norbut AM, Mendelow H. Primary glioblastoma multiforme of the pineal region with leptomeningeal metastases: a case report. Cancer. 1981; 47:592–596. PMID: 6261912.26. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The clinical features of spinal leptomeningeal dissemination from malignant gliomas. J Korean Neurosurg Soc. 2011; 49:334–338. PMID: 21887390.27. Zima LA, Tulpule S, Samson K, Shonka N. Seizure prevalence, contributing factors, and prognostic factors in patients with leptomeningeal disease. J Neurol Sci. 2019; 403:19–23. PMID: 31176194.28. Alatakis S, Malham GM, Thien C. Spinal leptomeningeal metastasis from cerebral glioblastoma multiforme presenting with radicular pain: case report and literature review. Surg Neurol. 2001; 56:33–37. discussion 37-8. PMID: 11546569.29. Chan DT, Hsieh SY, Kam MK, Cheung TC, Ng SC, Poon WS. Pattern of recurrence and factors associated with cerebrospinal fluid dissemination of glioblastoma in Chinese patients. Surg Neurol Int. 2016; 7:92. PMID: 27857856.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glioblastoma Multiforme in the Pineal Region with Leptomeningeal Dissemination and Lumbar Metastasis
- A Case of Meningioma Compatible with Metastatic Glioblastoma Multiforme
- A Case of Glioblastoma Multiforme of the Cerebellum
- A Case of Multicentric Glioblastoma Multiforme
- Scalp Metastasis of Glioblastoma Multiforme after Craniotomy and stereotatic Interstitial Brachytherapy