Acute Crit Care.
2022 May;37(2):137-150. 10.4266/acc.2021.01200.
Oxygen therapy for sepsis and prevention of complications
- Affiliations
-
- 1Independent researcher
- KMID: 2531670
- DOI: http://doi.org/10.4266/acc.2021.01200
Abstract
- Patients with sepsis have a wide range of respiratory disorders that can be treated with oxygen therapy. Experimental data in animal sepsis models show that oxygen therapy significantly increases survival, while clinical data on the use of different oxygen therapy protocols are ambiguous. Oxygen therapy, especially hyperbaric oxygenation, in patients with sepsis can aggravate existing oxidative stress and contribute to the development of disseminated intravascular coagulation. The purpose of this article is to compare experimental and clinical data on oxygen therapy in animals and humans, to discuss factors that can influence the results of oxygen therapy for sepsis treatment in humans, and to provide some recommendations for reducing oxidative stress and preventing disseminated intravascular coagulation during oxygen therapy.
Keyword
Figure
Reference
-
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020; 395:200–11.
Article2. Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O'Brien C, et al. Prevalence, Underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019; 2:e187571.
Article3. Byrne L, Van Haren F. Fluid resuscitation in human sepsis: time to rewrite history? Ann Intensive Care. 2017; 7:4.
Article4. Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019: results from a systematic review and meta-analysis. Crit Care. 2020; 24:239.
Article5. Schlapbach LJ, Kissoon N, Alhawsawi A, Aljuaid MH, Daniels R, Gorordo-Delsol LA, et al. World Sepsis Day: a global agenda to target a leading cause of morbidity and mortality. Am J Physiol Lung Cell Mol Physiol. 2020; 319:L518–22.
Article6. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013; 41:1167–74.
Article7. Klastrup V, Hvass AM, Mackenhauer J, Fuursted K, Schønheyder HC, Kirkegaard H, et al. Site of infection and mortality in patients with severe sepsis or septic shock. A cohort study of patients admitted to a Danish general intensive care unit. Infect Dis (Lond). 2016; 48:726–31.
Article8. Chou EH, Mann S, Hsu TC, Hsu WT, Liu CC, Bhakta T, et al. Incidence, trends, and outcomes of infection sites among hospitalizations of sepsis: a nationwide study. PLoS One. 2020; 15:e0227752.
Article9. Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007; 335:879–83.
Article10. Bewick T, Simmonds M, Chikhani M, Meyer J, Lim WS. Pneumonia in the context of severe sepsis: a significant diagnostic problem. Eur Respir J. 2008; 32:1417–8.
Article11. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014; 5:4–11.
Article12. Szabo BG, Kiss R, Lenart KS, Marosi B, Vad E, Lakatos B, et al. Clinical and microbiological characteristics and outcomes of community-acquired sepsis among adults: a single center, 1-year retrospective observational cohort study from Hungary. BMC Infect Dis. 2019; 19:584.
Article13. Vendemiato AV, von Nowakonski A, Marson FA, Levy CE. Microbiological characteristics of sepsis in a University hospital. BMC Infect Dis. 2015; 15:58.
Article14. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315:801–10.
Article15. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018; 392:75–87.
Article16. Weiss SL, Peters MJ, Alhazzani W, Agus M, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020; 21:e52–106.17. Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med. 2018; 44:1400–26.
Article18. Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018; 44:925–8.
Article19. Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med. 2020; 46:17–26.
Article20. Panditrao MM, Panditrao MM. Optimizing oxygen delivery in sepsis: a review. Adesh Univ J Med Sci Res. 2019; 1(1):8–15.
Article21. Heffner JE. The story of oxygen. Respir Care. 2013; 58:18–31.
Article22. Shultz SM, Hartmann PM. George E Holtzapple (1862-1946) and oxygen therapy for lobar pneumonia: the first reported case (1887) and a review of the contemporary literature to 1899. J Med Biogr. 2005; 13:201–6.
Article23. Ragaller M, Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 2010; 3:43–51.
Article24. Pham T, Rubenfeld GD. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017; 195:860–70.
Article25. Taylor MD, Tibby SM. Sometimes more is not always better: ScvO2 monitoring in pediatric sepsis. Intensive Care Med. 2020; 46:1264–6.
Article26. Bouvier NM. Animal models for influenza virus transmission studies: a historical perspective. CurrOpinVirol. 2015; 13:101–8.
Article27. Guillon A, Preau S, Aboab J, Azabou E, Jung B, Silva S, et al. Preclinical septic shock research: why we need an animal ICU. Ann Intensive Care. 2019; 9:66.
Article28. Bean AG, Baker ML, Stewart CR, Cowled C, Deffrasnes C, Wang LF, et al. Studying immunity to zoonotic diseases in the natural host: keeping it real. Nat Rev Immunol. 2013; 13:851–61.
Article29. Zingarelli B, Coopersmith CM, Drechsler S, Efron P, Marshall JC, Moldawer L, et al. Part I: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for study design and humane modeling endpoints. Shock. 2019; 51:10–22.
Article30. Thom SR, Lauermann MW, Hart GB. Intermittent hyperbaric oxygen therapy for reduction of mortality in experimental polymicrobial sepsis. J Infect Dis. 1986; 154:504–10.
Article31. Guven A, Gundogdu G, Vurucu S, Uysal B, Oztas E, Ozturk H, et al. Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2009; 44:1730–5.
Article32. Halbach JL, Prieto JM, Wang AW, Hawisher D, Cauvi DM, Reyes T, et al. Early hyperbaric oxygen therapy improves survival in a model of severe sepsis. Am J PhysiolRegulIntegr Comp Physiol. 2019; 317:R160–8.
Article33. Buras JA, Holt D, Orlow D, Belikoff B, Pavlides S, Reenstra WR. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit Care Med. 2006; 34:2624–9.
Article34. Aksenov I, Strauss M, Miller S, Truong C, Hart G. New roles of hyperbaric oxygen in sepsis. Chest. 2007; 132:557A.
Article35. Bektas A, Ulusoy M, Mas MR. Do late phase hyperbaric and normobaric oxygen therapies have effect on liver damage? An experimental sepsis model. Gen Med (Los Angel). 2019; 7:324.
Article36. Yamanel L, Kaldirim U, Oztas Y, Coskun O, Poyrazoglu Y, Durusu M, et al. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int J Med Sci. 2011; 8:48–55.
Article37. Hedetoft M, Garred P, Madsen MB, Hyldegaard O. Hyperbaric oxygen treatment is associated with a decrease in cytokine levels in patients with necrotizing soft-tissue infection. Physiol Rep. 2021; 9:e14757.
Article38. Nikitopoulou TS, Papalimperi AH. The inspiring journey of hyperbaric oxygen therapy, from the controversy to the acceptance by the scientific community. Health Sci J. 2015; 9:1.39. Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. A comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986; 121:191–5.40. Bærnthsen NF, Hansen MB, Wahl AM, Simonsen U, Hyldegaard O. Treatment with 24 h-delayed normo- and hyperbaric oxygenation in severe sepsis induced by cecal ligation and puncture in rats. J Inflamm (Lond). 2017; 14:27.41. Ozturk A, Yamanel L, Ozenc S, Ince M, Simsek K, Comert B, et al. Comparison of the effects of hyperbaric oxygen and normobaric oxygen on sepsis in rats. Arch Clin Exp Surg. 2016; 5:7–12.
Article42. Zhang Z, Ji X. Quadratic function between arterial partial oxygen pressure and mortality risk in sepsis patients: an interaction with simplified acute physiology score. Sci Rep. 2016; 6:35133.
Article43. Calzia E, Asfar P, Hauser B, Matejovic M, Ballestra C, Radermacher P, et al. Hyperoxia may be beneficial. Crit Care Med. 2010; 38(10 Suppl):S559–68.
Article44. Perner A, De Jong A, Shankar-Hari M. Trials on oxygen supplementation in sepsis: better late than never. Intensive Care Med. 2020; 46:116–8.
Article45. Muth CM, Radermacher P, Cuzzocrea S. Hyperbaric oxygen and sepsis: time to recognize. Intensive Care Med. 2005; 31:1150–2.
Article46. Imperatore F, Cuzzocrea S, Luongo C, Liguori G, Scafuro A, De Angelis A, et al. Hyperbaric oxygen therapy prevents vascular derangement during zymosan-induced multiple-organ-failure syndrome. Intensive Care Med. 2004; 30:1175–81.
Article47. Sakoda M, Ueno S, Kihara K, Arikawa K, Dogomori H, Nuruki K, et al. A potential role of hyperbaric oxygen exposure through intestinal nuclear factor-kappaB. Crit Care Med. 2004; 32:1722–9.48. Chang KY, Tsai PS, Huang TY, Wang TY, Yang S, Huang CJ. HO-1 mediates the effects of HBO pretreatment against sepsis. J Surg Res. 2006; 136:143–53.
Article49. Bar-Or D, Carrick MM, Mains CW, Rael LT, Slone D, Brody EN. Sepsis, oxidative stress, and hypoxia: are there clues to better treatment? Redox Rep. 2015; 20:193–7.
Article50. Chiumello D, Brioni M. Severe hypoxemia: which strategy to choose. Crit Care. 2016; 20:132.
Article51. Rossi P, Tauzin L, Weiss M, Rostain JC, Sainty JM, Boussuges A. Could hyperoxic ventilation impair oxygen delivery in septic patients? Clin Physiol Funct Imaging. 2007; 27:180–4.
Article52. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010; 55:40–6.
Article53. Dahl RM, Grønlykke L, Haase N, Holst LB, Perner A, Wetterslev J, et al. Variability in targeted arterial oxygenation levels in patients with severe sepsis or septic shock. Acta Anaesthesiol Scand. 2015; 59:859–69.
Article54. Hedenstierna G. Alveolar collapse and closure of airways: regular effects of anaesthesia. Clin Physiol Funct Imaging. 2003; 23:123–9.
Article55. Stolmeijer R, Bouma HR, Zijlstra JG, Drost-de Klerck AM, Ter Maaten JC, Ligtenberg J. A systematic review of the effects of hyperoxia in acutely ill patients: should we aim for less? Biomed Res Int. 2018; 2018:7841295.
Article56. Yumoto M, Nakamura F, Katsuya H. Septic shock following hyperbaric oxygen therapy in a patient with ileus. Jpn Soc Intensiv Care Med. 2004; 11:127–31.57. Vincent JL, Taccone FS, He X. Harmful effects of hyperoxia in postcardiac arrest, sepsis, traumatic brain injury, or stroke: the importance of individualized oxygen therapy in critically ill patients. Can Respir J. 2017; 2017:2834956.
Article58. Page D, Ablordeppey E, Wessman BT, Mohr NM, Trzeciak S, Kollef MH, et al. Emergency department hyperoxia is associated with increased mortality in mechanically ventilated patients: a cohort study. Crit Care. 2018; 22:9.
Article59. Ni YN, Wang YM, Liang BM, Liang ZA. The effect of hyperoxia on mortality in critically ill patients: a systematic review and meta analysis. BMC Pulm Med. 2019; 19:53.
Article60. Pannu SR. Too much oxygen: hyperoxia and oxygen management in mechanically ventilated patients. Semin Respir Crit Care Med. 2016; 37:16–22.
Article61. Damiani E, Donati A, Girardis M. Oxygen in the critically ill: friend or foe? Curr Opin Anaesthesiol. 2018; 31:129–35.62. Martin DS, Grocott MP. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med. 2013; 41:423–32.63. Jouffroy R, Saade A, Saint Martin LC, Philippe P, Carli P, Vivien B. Prognosis value of partial arterial oxygen pressure in patients with septic shock subjected to pre-hospital invasive ventilation. Am J Emerg Med. 2019; 37:56–60.
Article64. Polat G, Ugan RA, Cadirci E, Halici Z. Sepsis and septic shock: current treatment strategies and new approaches. Eurasian J Med. 2017; 49:53–8.
Article65. Garner WL, Downs JB, Reilley TE, Frolicher D, Kargi A, Fabri PJ. The effects of hyperoxia during fulminant sepsis. Surgery. 1989; 105:747–51.66. Siemieniuk R, Chu DK, Kim LH, Güell-Rous MR, Alhazzani W, Soccal PM, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018; 363:k4169.
Article67. Genga KR, Russell JA. Update of sepsis in the intensive care unit. J Innate Immun. 2017; 9:441–55.
Article68. Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015; 5:42.
Article69. Tuder RM, Hunt JM, Schmidt EP. Hyperoxia and apoptosis. Too much of a good thing? Am J Respir Crit Care Med. 2011; 183:964–5.
Article70. Rodgers JL, Iyer D, Rodgers LE, Vanthenapalli S, Panguluri SK. Impact of hyperoxia on cardiac pathophysiology. J Cell Physiol. 2019; 234:12595–603.
Article71. Escobar SJ, Slade JB Jr, Hunt TK, Cianci P. Adjuvant hyperbaric oxygen therapy (HBO2)for treatment of necrotizing fasciitis reduces mortality and amputation rate. Undersea Hyperb Med. 2005; 32:437–43.72. Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015; 2015:CD004123.
Article73. Chang YH, Chen PL, Tai MC, Chen CH, Lu DW, Chen JT. Hyperbaric oxygen therapy ameliorates the blood-retinal barrier breakdown in diabetic retinopathy. Clin Exp Ophthalmol. 2006; 34:584–9.
Article74. Piantadosi CA. Carbon monoxide poisoning. Undersea Hyperb Med. 2004; 31:167–77.
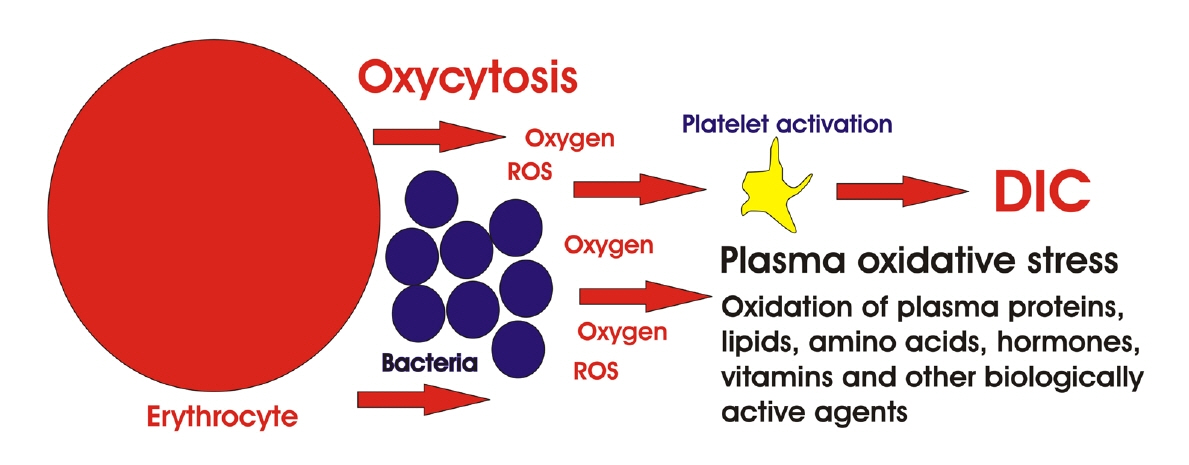
Article75. Minasyan H. Phagocytosis and oxycytosis: two arms of human innate immunity. Immunol Res. 2018; 66:271–80.
Article76. Minasyan H. Mechanisms and pathways for the clearance of bacteria from blood circulation in health and disease. Pathophysiology. 2016; 23:61–6.
Article77. Minasyan H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J Crit Care. 2017; 40:229–42.
Article78. Buico A, Cassino C, Ravera M, Betta PG, Osella D. Oxidative stress and total antioxidant capacity in human plasma. Redox Rep. 2009; 14:125–31.
Article79. Minasyan H. Sepsis: mechanisms of bacterial injury to the patient. Scand J Trauma Resusc Emerg Med. 2019; 27:19.
Article80. Krötz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004; 24:1988–96.81. Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. 2016; 2016:9060143.
Article82. Iuliano L, Colavita AR, Leo R, Praticò D, Violi F. Oxygen free radicals and platelet activation. Free Radic Biol Med. 1997; 22:999–1006.
Article83. Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018; 14:126–30.
Article84. Wachowicz B, Olas B, Zbikowska HM, Buczyński A. Generation of reactive oxygen species in blood platelets. Platelets. 2002; 13:175–82.
Article85. Sill JC, Proper JA, Johnson ME, Uhl CB, Katusic ZS. Reactive oxygen species and human platelet GP IIb/IIIa receptor activation. Platelets. 2007; 18:613–9.
Article86. Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013; 11:2148–54.
Article87. Davis RP, Miller-Dorey S, Jenne CN. Platelets and coagulation in infection. Clin Transl Immunology. 2016; 5:e89.
Article88. Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995; 25:843–51.
Article89. Alhamdi Y, Toh CH. Recent advances in pathophysiology of disseminated intravascular coagulation: the role of circulating histones and neutrophil extracellular traps. F1000Res. 2017; 6:2143.
Article90. Carrim N, Arthur JF, Hamilton JR, Gardiner EE, Andrews RK, Moran N, et al. Thrombin-induced reactive oxygen species generation in platelets: a novel role for protease-activated receptor 4 and GPIbα. Redox Biol. 2015; 6:640–7.
Article91. Kambas K, Mitroulis I, Ritis K. The emerging role of neutrophils in thrombosis-the journey of TF through NETs. Front Immunol. 2012; 3:385.
Article92. Delabranche X, Boisramé-Helms J, Asfar P, Berger A, Mootien Y, Lavigne T, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 2013; 39:1695–703.
Article93. Morrissey JH. Polyphosphate: a link between platelets, coagulation and inflammation. Int J Hematol. 2012; 95:346–52.
Article94. Arman M, Krauel K, Tilley DO, Weber C, Cox D, Greinacher A, et al. Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4. Blood. 2014; 123:3166–74.
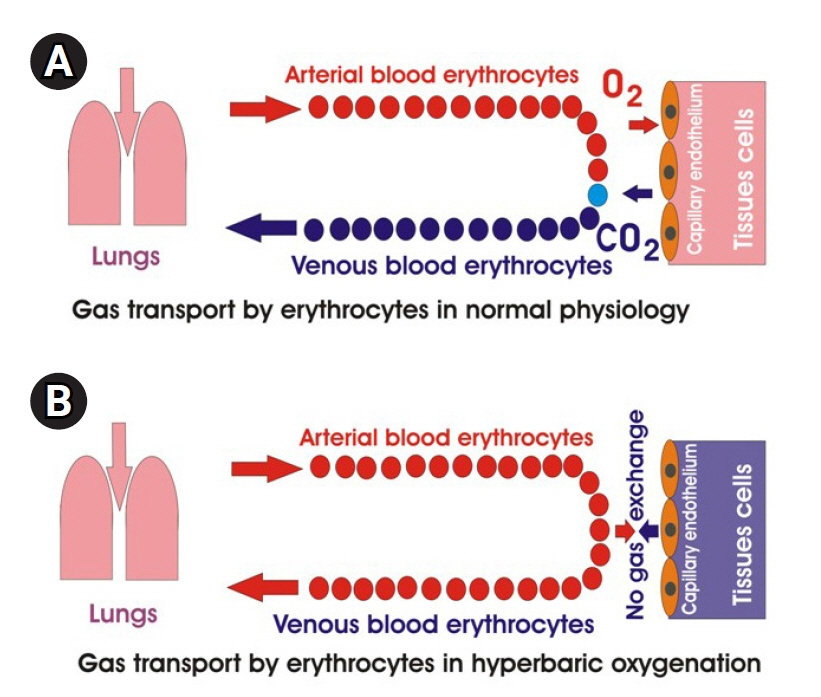
Article95. Antoniak S. The coagulation system in host defense. Res Pract ThrombHaemost. 2018; 2:549–57.
Article96. Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012; 129:290–5.
Article97. Broaddus VC. Murray and Nadel’s textbook of respiratory medicin. 6th ed. Philadelphia (PA): Elsevier Saunders;2016.98. Hafen B, Sharma S. Oxygen saturation [Internet]. StatPearls;2021. [cited 2021 Dec 10]. Available from: https://www.statpearls.com/kb/viewarticle/26491.99. Weibel ER. The pathway for oxygen. Cambridge: Harvard University Press;1984.100. Pittman RN. Oxygen transport. San Rafael (CA): Morgan and Claypool Life Sciences;2011. [cited 2021 Dec 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54103/.101. Collins JA, Rudenski A, Gibson J, Howard L, O'Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe (Sheff). 2015; 11:194–201.
Article102. Christmas KM, Bassingthwaighte JB. Equations for O2 and CO2 solubilities in saline and plasma: combining temperature and density dependences. J Appl Physiol (1985). 2017; 122:1313–20.103. Wojciechowski WV. Respiratory care sciences: an integrated approach. Clifton Park (NY): Delmar Cengage Learning;2014.104. Beachey W. Respiratory care anatomy and physiology: Foundations for Clinical Practice. St. Louis (MO): Elsevier;2018.105. Biolo G, Antonione R, De Cicco M. Glutathione metabolism in sepsis. Crit Care Med. 2007; 35(9 Suppl):S591–5.
Article106. Kim JS, Kwon WY, Suh GJ, Kim KS, Jung YS, Kim SH, et al. Plasma glutathione reductase activity and prognosis of septic shock. J Surg Res. 2016; 200:298–307.107. Gamarra Y, Santiago FC, Molina-López J, Castaño J, Herrera-Quintana L, Domínguez Á, et al. Pyroglutamic acidosis by glutathione regeneration blockage in critical patients with septic shock. Crit Care. 2019; 23:162.
Article108. Fläring U, Wernerman J. Glutathione in sepsis and multiple organ failure. In : Vincent JL, editor. Yearbook of intensive care and emergency medicine. Berlin, Heidelberg: Springer;2008. p. 444–53.109. Pravda J. Metabolic theory of septic shock. World J Crit Care Med. 2014; 3:45–54.
Article110. Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010; 9:3766–80.
Article111. Weber D, Davies MJ, Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol. 2015; 5:367–80.
Article112. Kuhn SO, Meissner K, Mayes LM, Bartels K. Vitamin C in sepsis. Curr Opin Anaesthesiol. 2018; 31:55–60.
Article113. Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017; 21:300.
Article114. Pakala R, Waksman R. Currently available methods for platelet function analysis: advantages and disadvantages. Cardiovasc Revasc Med. 2011; 12:312–22.
Article115. Layios N, Delierneux C, Hego A, Huart J, Gosset C, Lecut C, et al. Sepsis prediction in critically ill patients by platelet activation markers on ICU admission: a prospective pilot study. Intensive Care Med Exp. 2017; 5:32.
Article116. Zhou L, Schmaier AH. Platelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the field. Am J Clin Pathol. 2005; 123:172–83.
Article117. Collet JP, Montalescot G. Platelet function testing and implications for clinical practice. J Cardiovasc Pharmacol Ther. 2009; 14:157–69.
Article118. Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015; 11:133–48.
Article119. Darling CE, Sala Mercado JA, Quiroga-Castro W, Tecco GF, Zelaya FR, Conci EC, et al. Point-of-care assessment of platelet reactivity in the emergency department may facilitate rapid rule-out of acute coronary syndromes: a prospective cohort pilot feasibility study. BMJ Open. 2014; 4:e003883.
Article120. Fowler AA 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014; 12:32.
Article121. Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract. 2016; 5:94–100.
Article122. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017; 151:1229–38.
Article123. Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019; 322:1261–70.
Article124. Kalil AC. Lack of benefit of high-dose vitamin c, thiamine, and hydrocortisone combination for patients with sepsis. JAMA. 2020; 323:419–20.
Article125. de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, Spoelstra-de Man A, Girbes AR, Swart EL, et al. Vitamin C pharmacokinetics in critically ill patients: a randomized trial of four IV regimens. Chest. 2018; 153:1368–77.
Article126. Wang Y, Lin H, Lin BW, Lin JD. Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann Intensive Care. 2019; 9:58.
Article127. Spoelstra-de Man A, Oudemans-van Straaten HM, Berger MM. Adjuvant vitamin C for sepsis: mono or triple? Crit Care. 2019; 23:425.
Article128. Kashiouris MG, L'Heureux M, Cable CA, Fisher BJ, Leichtle SW, Fowler AA. The emerging role of vitamin C as a treatment for sepsis. Nutrients. 2020; 12:292.
Article129. Ribeiro Nogueira C, Ramalho A, Lameu E, Da Silva Franca CA, David C, Accioly E. Serum concentrations of vitamin A and oxidative stress in critically ill patients with sepsis. Nutr Hosp. 2009; 24:312–7.130. Zhang X, Yang K, Chen L, Liao X, Deng L, Chen S, et al. Vitamin A deficiency in critically ill children with sepsis. Crit Care. 2019; 23:267.
Article131. Cherukuri L, Gewirtz G, Osea K, Tayek JA. Vitamin A treatment for severe sepsis in humans; a prospective randomized double blind placebo-controlled clinical trial. Clin Nutr ESPEN. 2019; 29:49–51.
Article132. Hariri Ahari M, Pishbin E. Vitamin D and sepsis. Int Sch Res Notices. 2014; 1:225–8.133. Behera CK, Sahoo JP, Patra SD, Jena PK. Is lower vitamin D level associated with increased risk of neonatal sepsis? a prospective cohort study. Indian J Pediatr. 2020; 87:427–32.
Article134. Kempker JA, Han JE, Tangpricha V, Ziegler TR, Martin GS. Vitamin D and sepsis: an emerging relationship. Dermatoendocrinol. 2012; 4:101–8.135. Kempker JA, Martin GS. Vitamin D and sepsis: from associations to causal connections. Inflamm Allergy Drug Targets. 2013; 12:246–52.
Article136. Shojaei M, Sabzeghabaei A, Valaei Barhagh H, Soltani S. The correlation between serum level of vitamin D and outcome of sepsis patients: a cross-sectional study. Arch Acad Emerg Med. 2019; 7:e1.137. Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011; 15:R104.
Article138. Minter BE, Lowes DA, Webster NR, Galley HF. Differential effects of MitoVitE, α-tocopherol and trolox on oxidative stress, mitochondrial function and inflammatory signalling pathways in endothelial cells cultured under conditions mimicking sepsis. Antioxidants (Basel). 2020; 9:195.
Article139. Yamakawa K, Umemura Y, Murao S, Hayakawa M, Fujimi S. Optimal timing and early intervention with anticoagulant therapy for sepsis-induced disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2019; 25:1076029619835055.
Article140. Umemura Y, Yamakawa K, Hayakawa M, Hamasaki T, Fujimi S; Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) study group. Screening itself for disseminated intravascular coagulation may reduce mortality in sepsis: a nationwide multicenter registry in Japan. Thromb Res. 2018; 161:60–6.
Article141. Laursen MA, Larsen JB, Hvas AM. Platelet function in disseminated intravascular coagulation: a systematic review. Platelets. 2018; 29:238–48.
Article142. Assinger A, Schrottmaier WC, Salzmann M, Rayes J. Platelets in sepsis: an update on experimental models and clinical data. Front Immunol. 2019; 10:1687.
Article143. Kudo D, Hayakawa M, Iijima H, Yamakawa K, Saito S, Uchino S, et al. The treatment intensity of anticoagulant therapy for patients with sepsis-induced disseminated intravascular coagulation and outcomes: a multicenter cohort study. Clin Appl Thromb Hemost. 2019; 25:1076029619839154.
Article144. Dewitte A, Lepreux S, Villeneuve J, Rigothier C, Combe C, Ouattara A, et al. Blood platelets and sepsis pathophysiology: a new therapeutic prospect in critically [corrected] ill patients? Ann Intensive Care. 2017; 7:115.145. Lösche W, Boettel J, Kabisch B, Winning J, Claus RA, Bauer M. Do aspirin and other antiplatelet drugs reduce the mortality in critically ill patients? Thrombosis. 2012; 2012:720254.
Article146. Winning J, Neumann J, Kohl M, Claus RA, Reinhart K, Bauer M, et al. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit. Crit Care Med. 2010; 38:32–7.
Article147. Kelm DJ, Valerio-Rojas JC, Cabello-Garza J, Gajic O, Cartin-Ceba R. Predictors of disseminated intravascular coagulation in patients with septic shock. Int Sch Res Notices. 2013:219048.
Article148. Tsao CM, Ho ST, Wu CC. Coagulation abnormalities in sepsis. Acta Anaesthesiol Taiwan. 2015; 53:16–22.
Article149. Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. ThrombHaemost. 2009; 102:248–57.
Article150. Ouyang Y, Wang Y, Liu B, Ma X, Ding R. Effects of antiplatelet therapy on the mortality rate of patients with sepsis: a meta-analysis. J Crit Care. 2019; 50:162–168.
Article151. Wang Y, Ouyang Y, Liu B, Ma X, Ding R. Platelet activation and antiplatelet therapy in sepsis: a narrative review. Thromb Res. 2018; 166:28–36.
Article152. Senno SL, Pechet L, Bick RL. Disseminated intravascular coagulopathy (DIC): pathophysiology, laboratory diagnosis, and management. J Intensive Care Med. 2000; 15:144–58.
Article153. Sossdorf M, Otto GP, Boettel J, Winning J, Lösche W. Benefit of low-dose aspirin and non-steroidal anti-inflammatory drugs in septic patients. Crit Care. 2013; 17:402.
Article154. Eisen DP, Reid D, McBryde ES. Acetyl salicylic acid usage and mortality in critically ill patients with the systemic inflammatory response syndrome and sepsis. Crit Care Med. 2012; 40:1761–7.
Article155. Eisen DP. Manifold beneficial effects of acetyl salicylic acid and nonsteroidal anti-inflammatory drugs on sepsis. Intensive Care Med. 2012; 38:1249–57.
Article156. Otto GP, Sossdorf M, Boettel J, Kabisch B, Breuel H, Winning J, et al. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets. 2013; 24:480–5.
Article157. Eisen DP, Leder K, Woods RL, Lockery JE, McGuinness SL, Wolfe R, et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir Med. 2021; 9:186–95.
Article158. Evangelista V, Manarini S, Dell'Elba G, Martelli N, Napoleone E, Di Santo A, et al. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation. ThrombHaemost. 2005; 94:568–77.
Article159. Winning J, Reichel J, Eisenhut Y, Hamacher J, Kohl M, Deigner HP, et al. Anti-platelet drugs and outcome in severe infection: clinical impact and underlying mechanisms. Platelets. 2009; 20:50–7.
Article160. Dobesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014; 34:1077–90.
Article161. Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014; 25:517–25.
Article162. Scully M, Levi M. How we manage haemostasis during sepsis. Br J Haematol. 2019; 185:209–218.
Article163. Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001; 21:1094–6.
Article164. Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F, et al. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: a prospective clinical study. Exp Ther Med. 2014; 7:604–8.
Article165. Polderman KH, Girbes AR. Drug intervention trials in sepsis: divergent results. Lancet. 2004; 363:1721–3.
Article166. Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology. 2020; 132:1238–45.
Article167. Wang C, Chi C, Guo L, Wang X, Guo L, Sun J, et al. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: a systematic review and meta-analysis. Crit Care. 2014; 18:563.
Article