Int J Stem Cells.
2022 May;15(2):203-216. 10.15283/ijsc21178.
Diesel Exhaust Particles Impair Therapeutic Effect of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells against Experimental Colitis through ROS/ERK/cFos Signaling Pathway
- Affiliations
-
- 1Department of Biomedicine and Health Sciences, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Medical Life Sciences, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Agricultural Biotechnology and Research Institute of Agriculture and Life Sciences, Seoul National University, Seoul, Korea
- 4Bio-MAX Institute, Seoul National University, Seoul, Korea
- 5Division of Allergy and Respiratory Disease Research, Department of Chronic Disease Convergence Research, Korea National Institute of Health, Cheongju, Korea
- 6Biometrology Group, Korea Research Institute of Standards and Science (KRISS), Daejeon, Korea
- 7Department of Bio-Analytical Science, University of Science and Technology (UST), Daejeon, Korea
- 8Division of Nephrology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 9Laboratory of Clinical Immunology and Microbiology, NIAID, NIH, Bethesda, MD, USA
- 10Catholic High-Performance Cell Therapy Center & Department of Medical Life Science, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2529835
- DOI: http://doi.org/10.15283/ijsc21178
Abstract
- Background and Objectives
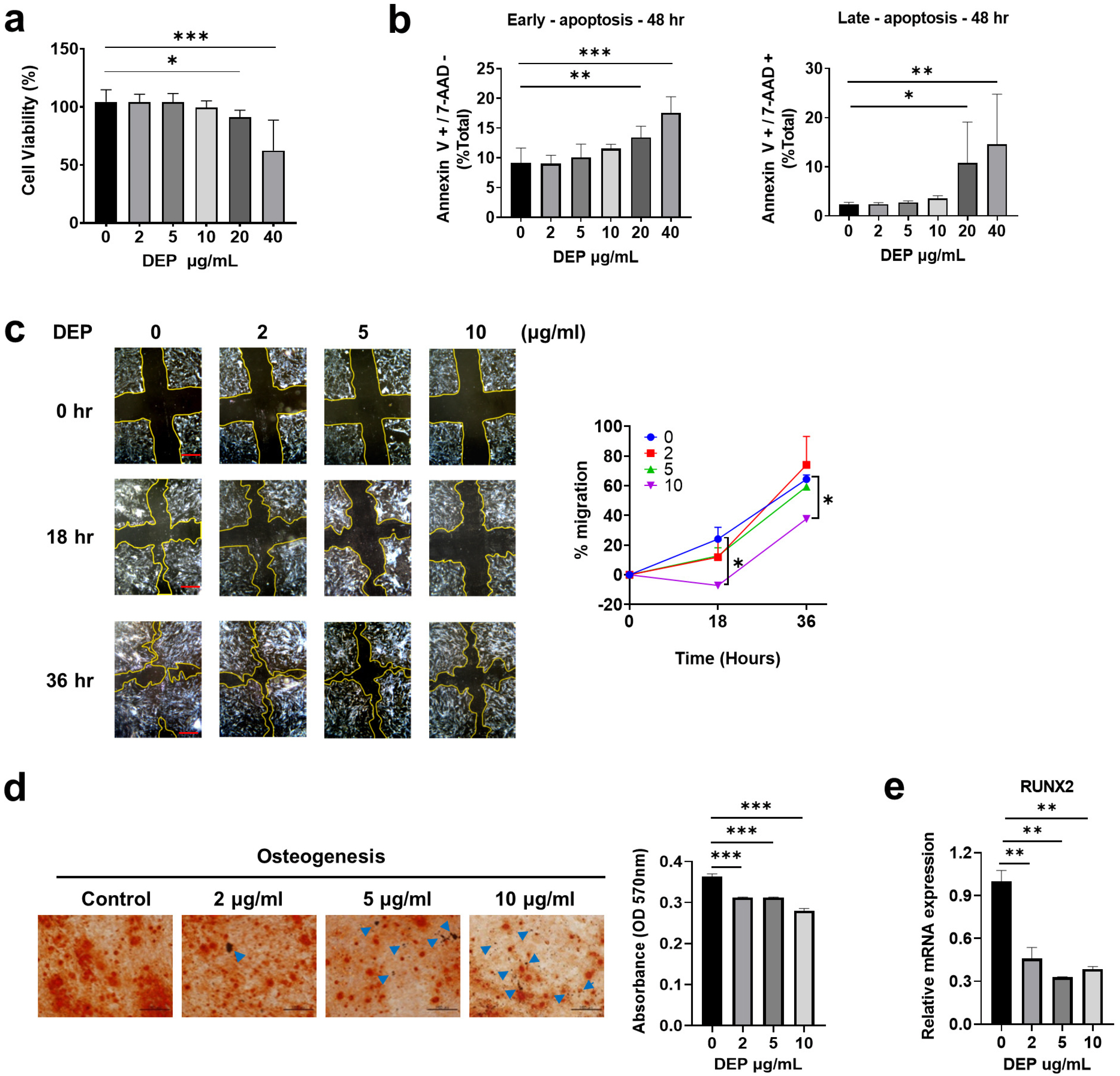
Epidemiological investigations have shown positive correlations between increased diesel exhaust particles (DEP) in ambient air and adverse health outcomes. DEP are the major constituent of particulate atmospheric pollution and have been shown to induce proinflammatory responses both in the lung and systemically. Here, we report the effects of DEP exposure on the properties of human Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs), including stemness, regeneration, and immunomodulation.
Methods and Results
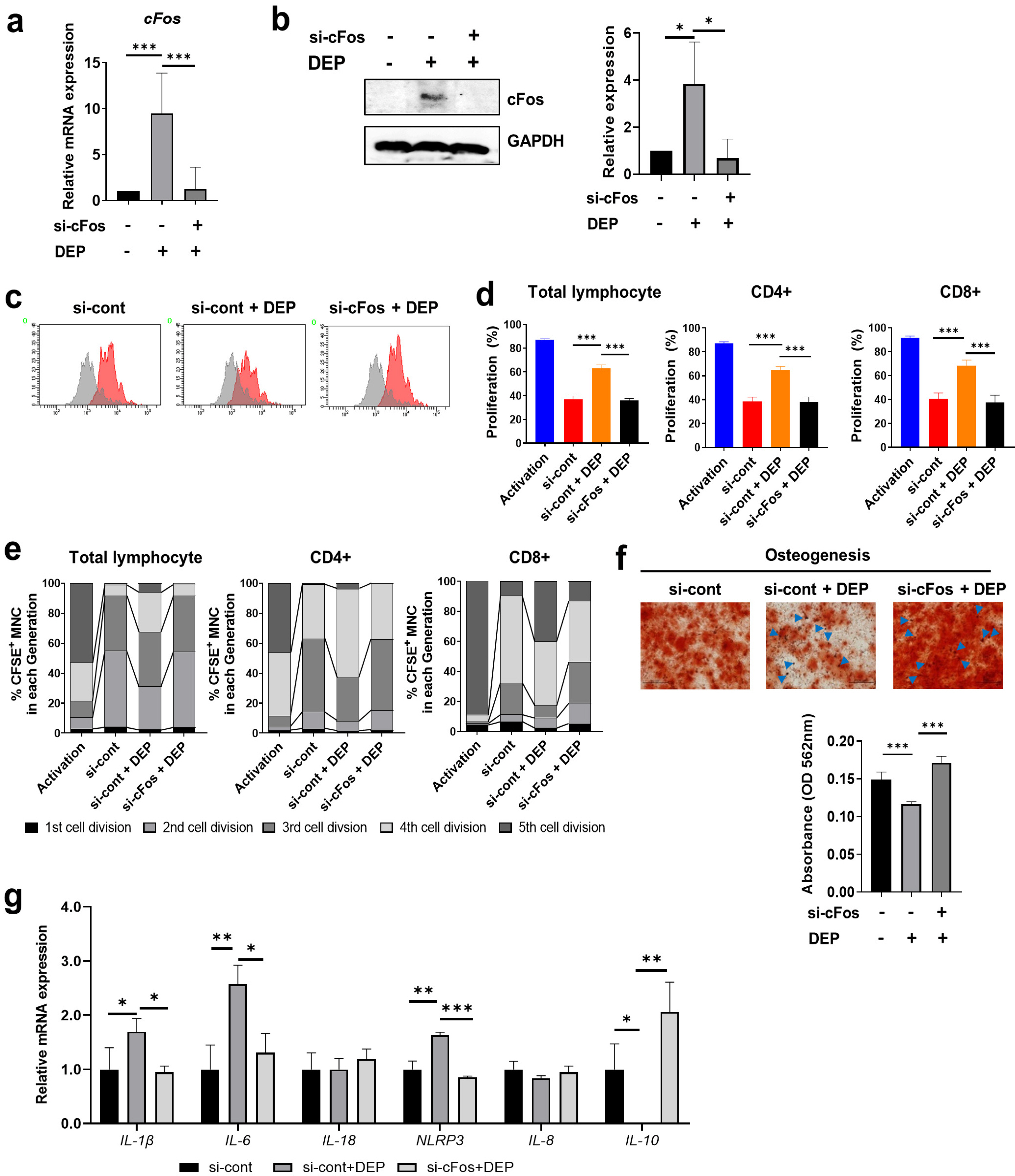
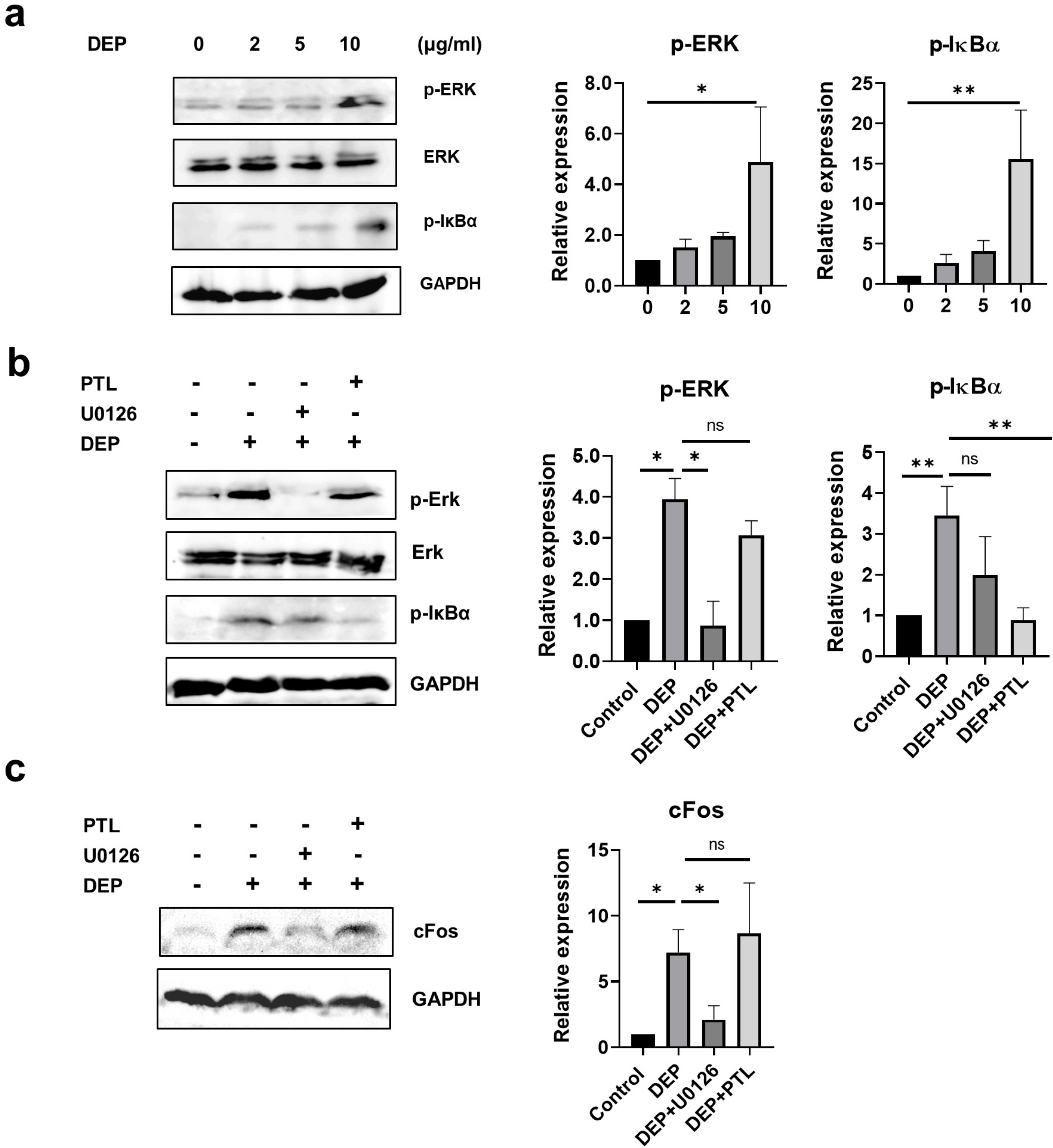
Non-apoptotic concentrations of DEP (10 μg/ml) inhibited the migration and osteogenic differentiation capacity of WJ-MSCs. Gene expression profiling showed that DEP increased intracellular reactive oxygen species (ROS) and expression of pro-inflammatory and metabolic-process-related genes including cFos. Furthermore, WJ-MSCs cultured with DEP showed impaired suppression of T cell proliferation that was reversed by inhibition of ROS or knockdown of cFos. ERK inhibition assay revealed that DEP-induced ROS regulated cFos through activation of ERK but not NF-κB signaling. Overall, low concentrations of DEP (10 μg/ml) significantly suppressed the stemness and immunomodulatory properties of WJ-MSCs through ROS/ERK/cFos signaling pathways. Furthermore, WJ-MSCs cultured with DEP impaired the therapeutic effect of WJ-MSCs in experimental colitis mice, but was partly reversed by inhibition of ROS.
Conclusions
Taken together, these results indicate that exposure to DEP enhances the expression of pro-inflammatory cytokines and immune responses through a mechanism involving the ROS/ERK/cFos pathway in WJ-MSCs, and that DEP-induced ROS damage impairs the therapeutic effect of WJ-MSCs in colitis. Our results suggest that modulation of ROS/ERK/cFos signaling pathways in WJ-MSCs might be a novel therapeutic strategy for DEP-induced diseases.
Keyword
Figure
Reference
-
References
1. Wilson SJ, Miller MR, Newby DE. 2018; Effects of diesel exhaust on cardiovascular function and oxidative stress. Antioxid Redox Signal. 28:819–836. DOI: 10.1089/ars.2017.7174. PMID: 28540736.
Article2. Li N, Hao M, Phalen RF, Hinds WC, Nel AE. 2003; Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 109:250–265. DOI: 10.1016/j.clim.2003.08.006. PMID: 14697739.3. van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. 2001; Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 164:826–830. DOI: 10.1164/ajrccm.164.5.2010160. PMID: 11549540.
Article4. Totlandsdal AI, Cassee FR, Schwarze P, Refsnes M, Låg M. 2010; Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part Fibre Toxicol. 7:41. DOI: 10.1186/1743-8977-7-41. PMID: 21162728. PMCID: PMC3012014.
Article5. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. 2008; Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 453:65–71. DOI: 10.1038/nature06880. PMID: 18362915.
Article6. Yu KR, Kang KS. 2013; Aging-related genes in mesenchymal stem cells: a mini-review. Gerontology. 59:557–563. DOI: 10.1159/000353857. PMID: 23970150.
Article7. Jimenez-Puerta GJ, Marchal JA, López-Ruiz E, Gálvez-Martín P. 2020; Role of mesenchymal stromal cells as therapeutic agents: potential mechanisms of action and implications in their clinical use. J Clin Med. 9:445. DOI: 10.3390/jcm9020445. PMID: 32041213. PMCID: PMC7074225. PMID: 8e180146d3c14e4abff7e71f9f50194e.
Article8. Yu KR, Lee JY, Kim HS, Hong IS, Choi SW, Seo Y, Kang I, Kim JJ, Lee BC, Lee S, Kurtz A, Seo KW, Kang KS. 2014; A p38 MAPK-mediated alteration of COX-2/PGE2 regulates immunomodulatory properties in human mesenchymal stem cell aging. PLoS One. 9:e102426. DOI: 10.1371/journal.pone.0102426. PMID: 25090227. PMCID: PMC4121064. PMID: bdbe3cd9c234493d9e6a578b070b4caa.
Article9. Song N, Scholtemeijer M, Shah K. 2020; Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 41:653–664. DOI: 10.1016/j.tips.2020.06.009. PMID: 32709406. PMCID: PMC7751844.
Article10. Kang JY, Oh MK, Joo H, Park HS, Chae DH, Kim J, Lee HR, Oh IH, Yu KR. 2020; Xeno-free condition enhances therapeutic functions of human Wharton's jelly-derived mesenchymal stem cells against experimental colitis by upregulated indoleamine 2,3-dioxygenase activity. J Clin Med. 9:2913. DOI: 10.3390/jcm9092913. PMID: 32927587. PMCID: PMC7565923. PMID: 4c9b305ade2548c1b4dbdc72577fe1b8.
Article11. Joo H, Oh MK, Kang JY, Park HS, Chae DH, Kim J, Lee JH, Yoo HM, Choi U, Kim DK, Lee H, Kim S, Yu KR. 2021; Extracellular vesicles from thapsigargin-treated mesenchymal stem cells ameliorated experimental colitis via enhanced immunomodulatory properties. Biomedicines. 9:209. DOI: 10.3390/biomedicines9020209. PMID: 33670708. PMCID: PMC7922639. PMID: 71f8c2c5c8464eb596ffdc5d4e4d28f1.
Article12. Fontaine MJ, Shih H, Schäfer R, Pittenger MF. 2016; Unraveling the mesenchymal stromal cells' paracrine immunomodulatory effects. Transfus Med Rev. 30:37–43. DOI: 10.1016/j.tmrv.2015.11.004. PMID: 26689863.
Article13. Kim SY, Oh SH, Lee JH, Suh MW, Park MK. 2019; Effects of placenta-derived mesenchymal stem cells on the particulate matter-induced damages in human middle ear epithelial cells. Stem Cells Int. 2019:4357684. DOI: 10.1155/2019/4357684. PMID: 31814835. PMCID: PMC6878801. PMID: 4c8da1debf8a4b9fa2df9a191274b56d.
Article14. Munir H, McGettrick HM. 2015; Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 24:2091–2100. DOI: 10.1089/scd.2015.0008. PMID: 26068030.
Article15. Trapnell C, Pachter L, Salzberg SL. 2009; TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 25:1105–1111. DOI: 10.1093/bioinformatics/btp120. PMID: 19289445. PMCID: PMC2672628.
Article16. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010; Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 28:511–515. DOI: 10.1038/nbt.1621. PMID: 20436464. PMCID: PMC3146043.
Article17. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012; Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. DOI: 10.1038/nprot.2012.016. PMID: 22383036. PMCID: PMC3334321.
Article18. Benjamini Y, Hochberg Y. 1995; Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 57:289–300. DOI: 10.1111/j.2517-6161.1995.tb02031.x.
Article19. O'Driscoll CA, Owens LA, Gallo ME, Hoffmann EJ, Afrazi A, Han M, Fechner JH, Schauer JJ, Bradfield CA, Mezrich JD. 2018; Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part Fibre Toxicol. 15:35. DOI: 10.1186/s12989-018-0271-3. PMID: 30143013. PMCID: PMC6109291.20. Meldrum K, Gant TW, Leonard MO. 2017; Diesel exhaust particulate associated chemicals attenuate expression of CXCL10 in human primary bronchial epithelial cells. Toxicol In Vitro. 45(Pt 3):409–416. DOI: 10.1016/j.tiv.2017.06.023. PMID: 28655636.
Article21. Raudoniute J, Stasiulaitiene I, Kulvinskiene I, Bagdonas E, Garbaras A, Krugly E, Martuzevicius D, Bironaite D, Aldonyte R. 2018; Pro-inflammatory effects of extracted urban fine particulate matter on human bronchial epithelial cells BEAS-2B. Environ Sci Pollut Res Int. 25:32277–32291. DOI: 10.1007/s11356-018-3167-8. PMID: 30225694.
Article22. Abu-Elmagd M, Alghamdi MA, Shamy M, Khoder MI, Costa M, Assidi M, Kadam R, Alsehli H, Gari M, Pushparaj PN, Kalamegam G, Al-Qahtani MH. 2017; Evaluation of the effects of airborne particulate matter on bone marrow-mesenchymal stem cells (BM-MSCs): cellular, molecular and systems biological approaches. Int J Environ Res Public Health. 14:440. DOI: 10.3390/ijerph14040440. PMID: 28425934. PMCID: PMC5409640.
Article23. Baeza-Squiban A, Bonvallot V, Boland S, Marano F. 1999; Diesel exhaust particles increase NF-kappaB DNA binding activity and c-FOS proto-oncogene expression in human bronchial epithelial cells. Toxicol In Vitro. 13:817–822. DOI: 10.1016/S0887-2333(99)00036-3. PMID: 20654555.
Article24. Pourazar J, Mudway IS, Samet JM, Helleday R, Blomberg A, Wilson SJ, Frew AJ, Kelly FJ, Sandström T. 2005; Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol. 289:L724–L730. DOI: 10.1152/ajplung.00055.2005. PMID: 15749742.
Article25. Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. 2005; Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2:10. DOI: 10.1186/1743-8977-2-10. PMID: 16242040. PMCID: PMC1280930.
Article26. Tseng CY, Wang JS, Chang YJ, Chang JF, Chao MW. 2015; Exposure to high-dose diesel exhaust particles induces intracellular oxidative stress and causes endothelial apoptosis in cultured in vitro capillary tube cells. Cardiovasc Toxicol. 15:345–354. DOI: 10.1007/s12012-014-9302-y. PMID: 25488805.
Article27. Luu NT, McGettrick HM, Buckley CD, Newsome PN, Rainger GE, Frampton J, Nash GB. 2013; Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem Cells. 31:2690–2702. DOI: 10.1002/stem.1511. PMID: 23939932.
Article28. Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. 2005; Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect. 113:1536–1541. DOI: 10.1289/ehp.8094. PMID: 16263508. PMCID: PMC1310915.
Article29. Li R, Kou X, Xie L, Cheng F, Geng H. 2015; Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ Sci Pollut Res Int. 22:20167–20176. DOI: 10.1007/s11356-015-5222-z. PMID: 26304807.
Article30. Lee BC, Yu KR. 2020; Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 53:65–73. DOI: 10.5483/BMBRep.2020.53.2.291. PMCID: PMC7061209. PMID: 31964472.
Article31. Mamessier E, Nieves A, Vervloet D, Magnan A. 2006; Diesel exhaust particles enhance T-cell activation in severe asthmatics. Allergy. 61:581–588. DOI: 10.1111/j.1398-9995.2006.01056.x. PMID: 16629788.
Article32. Abarrategi A, Gambera S, Alfranca A, Rodriguez-Milla MA, Perez-Tavarez R, Rouault-Pierre K, Waclawiczek A, Chakravarty P, Mulero F, Trigueros C, Navarro S, Bonnet D, García-Castro J. 2018; c-Fos induces chondrogenic tumor formation in immortalized human mesenchymal progenitor cells. Sci Rep. 8:15615. DOI: 10.1038/s41598-018-33689-0. PMID: 30353072. PMCID: PMC6199246.
Article33. Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, Baeza-Squiban A. 2003; Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 285:L671–L679. DOI: 10.1152/ajplung.00419.2002. PMID: 12730081.
Article34. Tal TL, Simmons SO, Silbajoris R, Dailey L, Cho SH, Ramabhadran R, Linak W, Reed W, Bromberg PA, Samet JM. 2010; Differential transcriptional regulation of IL-8 expression by human airway epithelial cells exposed to diesel exhaust particles. Toxicol Appl Pharmacol. 243:46–54. DOI: 10.1016/j.taap.2009.11.011. PMID: 19914270.
Article35. Hendryx M, Luo J. 2020; COVID-19 prevalence and fatality rates in association with air pollution emission concentrations and emission sources. Environ Pollut. 265(Pt A):115126. DOI: 10.1016/j.envpol.2020.115126. PMID: 32806422. PMCID: PMC7320861.
Article36. Baulig A, Blanchet S, Rumelhard M, Lacroix G, Marano F, Baeza-Squiban A. 2007; Fine urban atmospheric particulate matter modulates inflammatory gene and protein expression in human bronchial epithelial cells. Front Biosci. 12:771–782. DOI: 10.2741/2100. PMID: 17127337.
Article37. Paital B, Agrawal PK. 2021; Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environ Chem Lett. 19:25–42. DOI: 10.1007/s10311-020-01091-w. PMID: 32982622. PMCID: PMC7499935.
Article38. Avanzini MA, Mura M, Percivalle E, Bastaroli F, Croce S, Valsecchi C, Lenta E, Nykjaer G, Cassaniti I, Bagnarino J, Baldanti F, Zecca M, Comoli P, Gnecchi M. 2021; Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl Med. 10:636–642. DOI: 10.1002/sctm.20-0385. PMID: 33188579. PMCID: PMC7753681. PMID: 08ada19240c5488898aa18a5c7b10b2c.
Article39. Lee BC, Kang I, Yu KR. 2021; Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes. J Clin Med. 10:711. DOI: 10.3390/jcm10040711. PMID: 33670202. PMCID: PMC7916919. PMID: 7d5a058402dc48c1940d63d771b7d7c5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential
- Increase of diesel car raises health risk in spite of recent development in engine technology
- Differentiation of human male germ cells from Wharton's jelly-derived mesenchymal stem cells
- Cardioprotective Effects of Wharton Jelly Derived Mesenchymal Stem Cell Transplantation in a Rodent Model of Myocardial Injury
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells