J Korean Med Sci.
2022 May;37(19):e154. 10.3346/jkms.2022.37.e154.
Microscopic Polyangiitis Following mRNA COVID-19 Vaccination: A Case Report
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea
- 2Department of Pathology, Hanyang University Guri Hospital, Guri, Korea
- 3Department of Pathology, Hanyang University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2529777
- DOI: http://doi.org/10.3346/jkms.2022.37.e154
Abstract
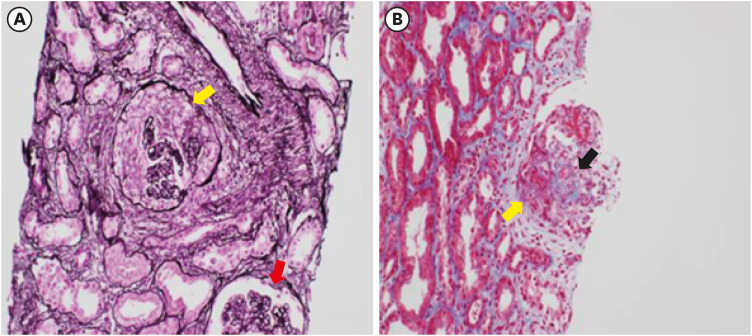
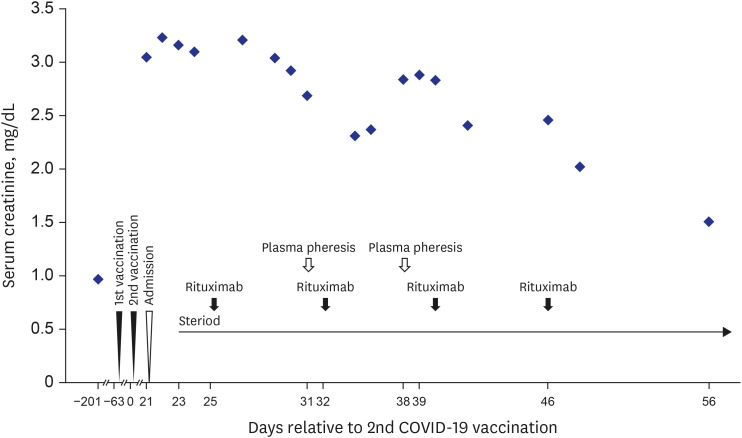
- Coronavirus disease 2019 (COVID-19) is one of the most widespread viral infections in human history. As a breakthrough against infection, vaccines have been developed to achieve herd immunity. Here, we report the first case of microscopic polyangiitis (MPA) following BNT162b2 vaccination in Korea. A 42-year-old man presented to the emergency room with general weakness, dyspnea, and edema after the second BNT162b2 vaccination. He had no medical history other than being treated for tuberculosis last year. Although his renal function was normal at last year, acute kidney injury was confirmed at the time of admission to the emergency room. His serum creatinine was 3.05 mg/dL. Routine urinalysis revealed proteinuria (3+) and hematuria. When additional tests were performed for suspected glomerulonephritis, the elevation of myeloperoxidase (MPO) antibody (38.6 IU/mL) was confirmed. Renal biopsy confirmed pauci-immune anti-neutrophil cytoplasmic antibody (ANCA)-related glomerulonephritis and MPA was diagnosed finally. As an induction therapy, a combination of glucocorticoid and rituximab was administered, and plasmapheresis was performed twice. He was discharged after the induction therapy and admitted to the outpatient clinic 34 days after induction therapy. During outpatient examination, his renal function had improved with serum creatinine 1.51 mg/dL. We suggest that MPA needs to be considered if patients have acute kidney injury, proteinuria, and hematuria after vaccination.
Keyword
Figure
Reference
-
1. WHO coronavirus (COVID-19) dashboard. Accessed November 29, 2021. https://covid19.who.int/ .2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020; 5(4):536–544. PMID: 32123347.3. Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022; 20(4):193–205. PMID: 34646006.4. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022; 399(10328):924–944. PMID: 35202601.5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.6. Choi YY, Kim MK, Kwon HC, Kim GH. Safety monitoring after the BNT162b2 COVID-19 vaccine among adults aged 75 years or older. J Korean Med Sci. 2021; 36(45):e318. PMID: 34811980.7. Cohen SR, Gao DX, Kahn JS, Rosmarin D. Comparison of constitutional and dermatologic side effects between COVID-19 and non-COVID-19 vaccines: review of a publicly available database of vaccine side effects. J Am Acad Dermatol. 2022; 86(1):248–249. PMID: 34592382.8. Birck R, Kaelsch I, Schnuelle P, Flores-Suárez LF, Nowack R. ANCA-associated vasculitis following influenza vaccination: causal association or mere coincidence? J Clin Rheumatol. 2009; 15(6):289–291. PMID: 19734734.9. Duggal T, Segal P, Shah M, Carter-Monroe N, Manoharan P, Geetha D. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol. 2013; 38(2):174–178. PMID: 23941822.10. Yanai-Berar N, Ben-Itzhak O, Gree J, Nakhoul F. Influenza vaccination induced leukocytoclastic vasculitis and pauci-immune crescentic glomerulonephritis. Clin Nephrol. 2002; 58(3):220–223. PMID: 12356192.11. Watanabe T. Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017; 13(3):188–196. PMID: 28521688.12. Bonetto C, Trotta F, Felicetti P, Alarcón GS, Santuccio C, Bachtiar NS, et al. Vasculitis as an adverse event following immunization - systematic literature review. Vaccine. 2016; 34(51):6641–6651. PMID: 26398442.13. Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. 2021; 6(12):3087–3089. PMID: 34423176.14. Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol. 2021; 12:762006. PMID: 34659268.15. Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021; 78(4):611–613. PMID: 34280507.16. Obata S, Hidaka S, Yamano M, Yanai M, Ishioka K, Kobayashi S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin Kidney J. 2021; 15(2):357–359. PMID: 35140936.17. Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021; 100(2):473–474. PMID: 34081948.18. Jeffs LS, Peh CA, Jose MD, Lange K, Hurtado PR. Randomized trial investigating the safety and efficacy of influenza vaccination in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2015; 20(5):343–351. PMID: 25656094.19. Rihova Z, Jancova E, Merta M, Rysava R, Reiterova J, Zabka J, et al. Long-term outcome of patients with antineutrophil cytoplasmic autoantibody-associated vasculitis with renal involvement. Kidney Blood Press Res. 2005; 28(3):144–152. PMID: 15908752.20. Day CJ, Howie AJ, Nightingale P, Shabir S, Adu D, Savage CO, et al. Prediction of ESRD in pauci-immune necrotizing glomerulonephritis: quantitative histomorphometric assessment and serum creatinine. Am J Kidney Dis. 2010; 55(2):250–258. PMID: 20045237.21. Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003; 41(4):776–784. PMID: 12666064.22. Tomilina NA, Biriukova LS, Egorova ET, Sukhanov AV, Stoliarevich ES, Kupavtseva OA, et al. Rapidly progressive glomerulonephritis in ANCA-associated vasculitis: a course, treatment efficacy, prognosis. Ter Arkh. 2008; 80(6):15–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eosinophilic Granulomatosis With Polyangiitis Following COVID-19 Vaccination: A Case Report
- Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
- Ipsilateral Radial Neuropathy after COVID-19 mRNA Vaccination in an Immunocompetent Young Man
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- mRNA COVID-19 Vaccine-Associated Subserosal Eosinophilic Gastroenteritis: A Case Report