Endocrinol Metab.
2022 Apr;37(2):249-260. 10.3803/EnM.2021.1235.
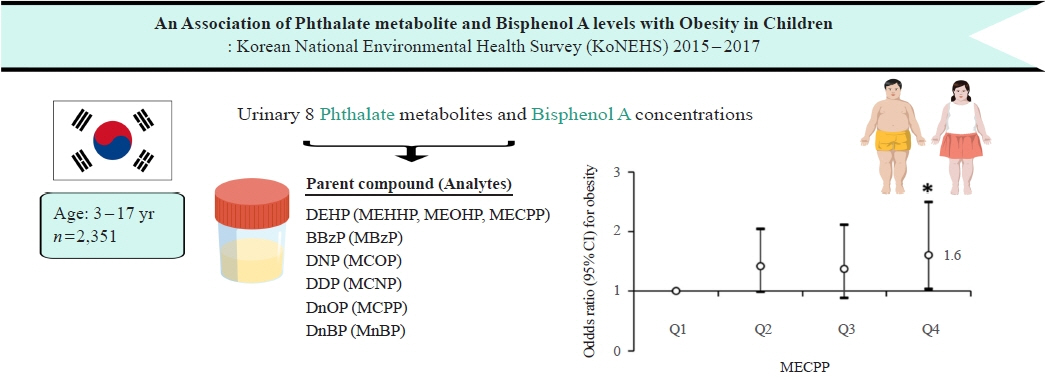
Associations of Phthalate Metabolites and Bisphenol A Levels with Obesity in Children: The Korean National Environmental Health Survey (KoNEHS) 2015 to 2017
- Affiliations
-
- 1Department of Pediatrics, Wonjin Green Hospital, Seoul, Korea
- 2Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 3Department of Pediatrics, Inje University Sanggye Paik Hospital, College of Medicine, Inje University, Seoul, Korea
- KMID: 2529217
- DOI: http://doi.org/10.3803/EnM.2021.1235
Abstract
- Background
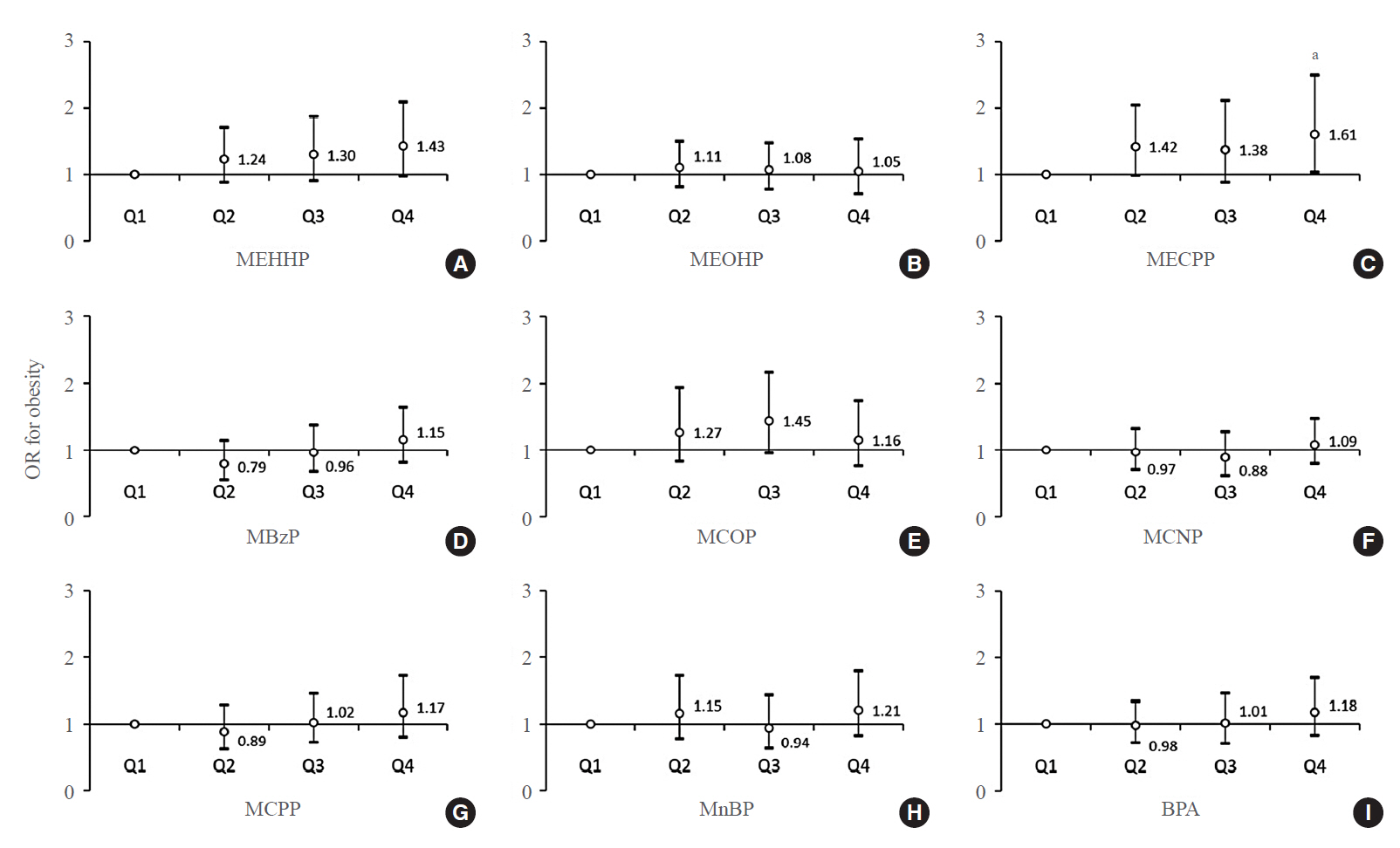
Phthalates and bisphenol A (BPA) are synthetic chemicals widely used in daily life. This study investigated urinary phthalate and BPA levels in Korean children and their associations with obesity. Methods: A total of 2,351 children aged 3 to 17 years who participated in the Korean National Environmental Health Survey 2015 to 2017 were included. Urinary dilution was corrected using covariate-adjusted standardization (CAS). We examined the geometric mean (GM) concentrations of urinary phthalate metabolites, including di (2-ethylhexyl) phthalate (DEHP) metabolites (mono [2-ethyl-5-hydroxyhexyl] phthalate, mono [2-ethyl-5-oxohexyl] phthalate, and mono [2-ethyl-5-carboxypentyl] phthalate [MECPP]), mono-benzyl-phthalate (MBzP), mono (carboxyoctyl) phthalate (MCOP), mono (carboxy-isononyl) phthalate (MCNP), mono (3-carboxypropyl) phthalate, and mono-n-butyl-phthalate (MnBP), and BPA. We also analyzed the odds ratio (OR) for obesity according to the quartiles of each analyte. Results: The urinary GM levels of DEHP metabolites and MnBP were notably higher among Korean children than among American, Canadian, and German children. The CAS-applied GM concentrations of most analytes, except for MBzP, MCOP, and MCNP, were higher in children aged 3 to 5 years than in those aged 6 to 17 years. The OR for obesity in the highest quartile of MECPP was significantly higher than in the lowest quartile after adjusting for covariates. However, the other phthalate metabolites and BPA were not significantly associated with obesity. Conclusion: The concentrations of urinary DEHP metabolites and MnBP were higher in Korean children than in children in Western countries. Urinary MECPP exposure, but not other phthalates or BPA, showed a positive association with obesity in Korean children. Further studies are required to elucidate the causal relationships.
Keyword
Figure
Reference
-
1. Ribeiro C, Mendes V, Peleteiro B, Delgado I, Araujo J, Aggerbeck M, et al. Association between the exposure to phthalates and adiposity: a meta-analysis in children and adults. Environ Res. 2019; 179(Pt A):108780.
Article2. Golestanzadeh M, Riahi R, Kelishadi R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2019; 26:35670–86.
Article3. Kim SH, Park MJ. Phthalate exposure and childhood obesity. Ann Pediatr Endocrinol Metab. 2014; 19:69–75.
Article4. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36:E1–150.
Article5. Kim MJ, Park YJ. Bisphenols and thyroid hormone. Endocrinol Metab (Seoul). 2019; 34:340–8.
Article6. Stojanoska MM, Milosevic N, Milic N, Abenavoli L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine. 2017; 55:666–81.
Article7. Kim KY, Lee E, Kim Y. The association between bisphenol A exposure and obesity in children: a systematic review with meta-analysis. Int J Environ Res Public Health. 2019; 16:2521.
Article8. Moon S, Seo MY, Choi K, Chang YS, Kim SH, Park MJ. Urinary bisphenol A concentrations and the risk of obesity in Korean adults. Sci Rep. 2021; 11:1603.
Article9. O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016; 124:220–7.
Article10. Bulka CM, Mabila SL, Lash JP, Turyk ME, Argos M. Arsenic and obesity: a comparison of urine dilution adjustment methods. Environ Health Perspect. 2017; 125:087020.
Article11. Hwang M, Choi K, Park C. Urinary levels of phthalate, bisphenol, and paraben and allergic outcomes in children: Korean National Environmental Health Survey 2015-2017. Sci Total Environ. 2022; 818:151703.
Article12. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article13. Geller RJ, Brotman RM, O’Brien KM, Fine DM, Zota AR. Phthalate exposure and odds of bacterial vaginosis among U.S. reproductive-aged women, NHANES 2001-2004. Reprod Toxicol. 2018; 82:1–9.
Article14. Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals 2019 [Internet]. Atlanta: CDC;2019. [cited 2022 Mar 21]. Available from: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf.15. Health Canada. Fifth Report on Human Biomonitoring of Environmental Chemicals in Canada: results of the Canadian Health Measures Survey Cycle 5 (2016-2017) [Internet]. Ottawa: Health Canada;2019. [cited 2022 Mar 21]. Available from: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/fifth-report-human-biomonitoring.html.16. Schwedler G, Rucic E, Lange R, Conrad A, Koch HM, Palmke C, et al. Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014-2017. Int J Hyg Environ Health. 2020; 225:113444.
Article17. Park C, Hwang M, Baek Y, Jung S, Lee Y, Paek D, et al. Urinary phthalate metabolite and bisphenol A levels in the Korean adult population in association with sociodemographic and behavioral characteristics: Korean National Environmental Health Survey (KoNEHS) 2012-2014. Int J Hyg Environ Health. 2019; 222:903–10.
Article18. Jeon S, Kim KT, Choi K. Migration of DEHP and DINP into dust from PVC flooring products at different surface temperature. Sci Total Environ. 2016; 547:441–6.
Article19. Ding S, Zhang Z, Chen Y, Qi W, Zhang Y, Xu Q, et al. Urinary levels of phthalate metabolites and their association with lifestyle behaviors in Chinese adolescents and young adults. Ecotoxicol Environ Saf. 2019; 183:109541.
Article20. Lee J, Lee JH, Kim CK, Thomsen M. Childhood exposure to DEHP, DBP and BBP under existing chemical management systems: a comparative study of sources of childhood exposure in Korea and in Denmark. Environ Int. 2014; 63:77–91.
Article21. Lehmler HJ, Liu B, Gadogbe M, Bao W. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: the National Health and Nutrition Examination Survey 2013-2014. ACS Omega. 2018; 3:6523–32.
Article22. Liao C, Liu W, Zhang J, Shi W, Wang X, Cai J, et al. Associations of urinary phthalate metabolites with residential characteristics, lifestyles, and dietary habits among young children in Shanghai, China. Sci Total Environ. 2018; 616-617:1288–97.
Article23. Lv Z, Cheng J, Huang S, Zhang Y, Wu S, Qiu Y, et al. DEHP induces obesity and hypothyroidism through both central and peripheral pathways in C3H/He mice. Obesity (Silver Spring). 2016; 24:368–78.
Article24. Yilmaz B, Terekeci H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord. 2020; 21:127–47.
Article25. Su H, Yuan P, Lei H, Zhang L, Deng D, Zhang L, et al. Long-term chronic exposure to di-(2-ethylhexyl)-phthalate induces obesity via disruption of host lipid metabolism and gut microbiota in mice. Chemosphere. 2022; 287(Pt 4):132414.
Article26. Fan Y, Qin Y, Chen M, Li X, Wang R, Huang Z, et al. Prenatal low-dose DEHP exposure induces metabolic adaptation and obesity: role of hepatic thiamine metabolism. J Hazard Mater. 2020; 385:121534.
Article27. Chen MY, Liu HP, Cheng J, Chiang SY, Liao WP, Lin WY. Transgenerational impact of DEHP on body weight of Drosophila. Chemosphere. 2019; 221:493–9.
Article28. Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diabetes. 2014; 4:e115.
Article29. Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect. 2012; 120:1123–9.
Article30. Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012; 32:619–29.31. Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ Health Perspect. 2013; 121:501–6.
Article32. Deierlein AL, Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez MP, et al. Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology. 2016; 27:492–9.
Article33. Xia B, Zhu Q, Zhao Y, Ge W, Zhao Y, Song Q, et al. Phthalate exposure and childhood overweight and obesity: urinary metabolomic evidence. Environ Int. 2018; 121(Pt 1):159–68.
Article34. Ashley-Martin J, Dodds L, Arbuckle TE, Lanphear B, Muckle G, Foster WG, et al. Urinary phthalates and body mass index in preschool children: the MIREC Child Development Plus study. Int J Hyg Environ Health. 2021; 232:113689.
Article35. On J, Kim SH, Lee J, Park MJ, Lee SW, Pyo H. Urinary di(2-ethylhexyl)phthalate metabolite ratios in obese children of South Korea. Environ Sci Pollut Res Int. 2021; 28:29590–600.
Article36. Kim SH, On JW, Pyo H, Ko KS, Won JC, Yang J, et al. Percentage fractions of urinary di(2-ethylhexyl) phthalate metabolites: association with obesity and insulin resistance in Korean girls. PLoS One. 2018; 13:e0208081.
Article37. Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, et al. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018; 211:547–56.
Article38. Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007-2010. Int J Hyg Environ Health. 2014; 217:687–94.
Article39. Zhang Y, Meng X, Chen L, Li D, Zhao L, Zhao Y, et al. Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS One. 2014; 9:e104852.
Article40. Wu W, Wu P, Yang F, Sun DL, Zhang DX, Zhou YK. Association of phthalate exposure with anthropometric indices and blood pressure in first-grade children. Environ Sci Pollut Res Int. 2018; 25:23125–34.
Article41. Lien GW, Chen JH, Tien FW, Chen PC, Chen HW, Hwa HL, et al. Dilute-and-shoot enhances sensitivity of phthalate urinary concentrations for assessing the exposure in children. J Hazard Mater. 2018; 351:124–30.
Article42. Jacobson MH, Woodward M, Bao W, Liu B, Trasande L. Urinary bisphenols and obesity prevalence among U.S. children and adolescents. J Endocr Soc. 2019; 3:1715–26.
Article43. Kasper-Sonnenberg M, Koch HM, Wittsiepe J, Bruning T, Wilhelm M. Phthalate metabolites and bisphenol A in urines from German school-aged children: results of the Duisburg birth cohort and Bochum cohort studies. Int J Hyg Environ Health. 2014; 217:830–8.
Article44. Xue J, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res. 2015; 137:120–8.
Article45. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012; 308:1113–21.
Article46. Eng DS, Lee JM, Gebremariam A, Meeker JD, Peterson K, Padmanabhan V. Bisphenol A and chronic disease risk factors in US children. Pediatrics. 2013; 132:e637–45.
Article47. Mustieles V, Casas M, Ferrando-Marco P, Ocon-Hernandez O, Reina-Perez I, Rodriguez-Carrillo A, et al. Bisphenol A and adiposity measures in peripubertal boys from the INMA-Granada cohort. Environ Res. 2019; 173:443–51.
Article48. D’Aniello R, Troisi J, D’Amico O, Sangermano M, Massa G, Moccaldo A, et al. Emerging pathomechanisms involved in obesity. J Pediatr Gastroenterol Nutr. 2015; 60:113–9.
Article49. Wang HX, Zhou Y, Tang CX, Wu JG, Chen Y, Jiang QW. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health. 2012; 11:79.
Article50. Lee I, Park YJ, Kim MJ, Kim S, Choi S, Park J, et al. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Environ Int. 2021; 146:106227.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between urinary phthalate metabolites and obesity in adult Korean population: Korean National Environmental Health Survey (KoNEHS), 2012–2014
- Determination of Phthalate Metabolites in Human Serum and Urine as Biomarkers for Phthalate Exposure Using Column-Switching LC-MS/MS
- Relationship between dietary factors and bisphenol a exposure: the second Korean National Environmental Health Survey (KoNEHS 2012–2014)
- Associations Between Thyroid Hormone Levels and Urinary Concentrations of Bisphenol A, F, and S in 6-Year-old Children in Korea
- Phthalate exposure and childhood obesity