Cancer Res Treat.
2022 Apr;54(2):505-516. 10.4143/crt.2020.1198.
Real-World Efficacy Data and Predictive Clinical Parameters for Treatment Outcomes in Advanced Esophageal Squamous Cell Carcinoma Treated with Immune Checkpoint Inhibitors
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Oncology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2528220
- DOI: http://doi.org/10.4143/crt.2020.1198
Abstract
- Purpose
This study aimed to evaluate the real-world efficacy of immune checkpoint inhibitors (ICIs), and to identify clinicolaboratory factors to predict treatment outcomes in patients with advanced esophageal squamous cell carcinoma (ESCC) receiving ICIs.
Materials and Methods
Sixty patients with metastatic or unresectable ESCC treated with nivolumab (n=48) or pembrolizumab (n=12) as ≥ second-line treatment between 2016 and 2019 at Asan Medical Center were included.
Results
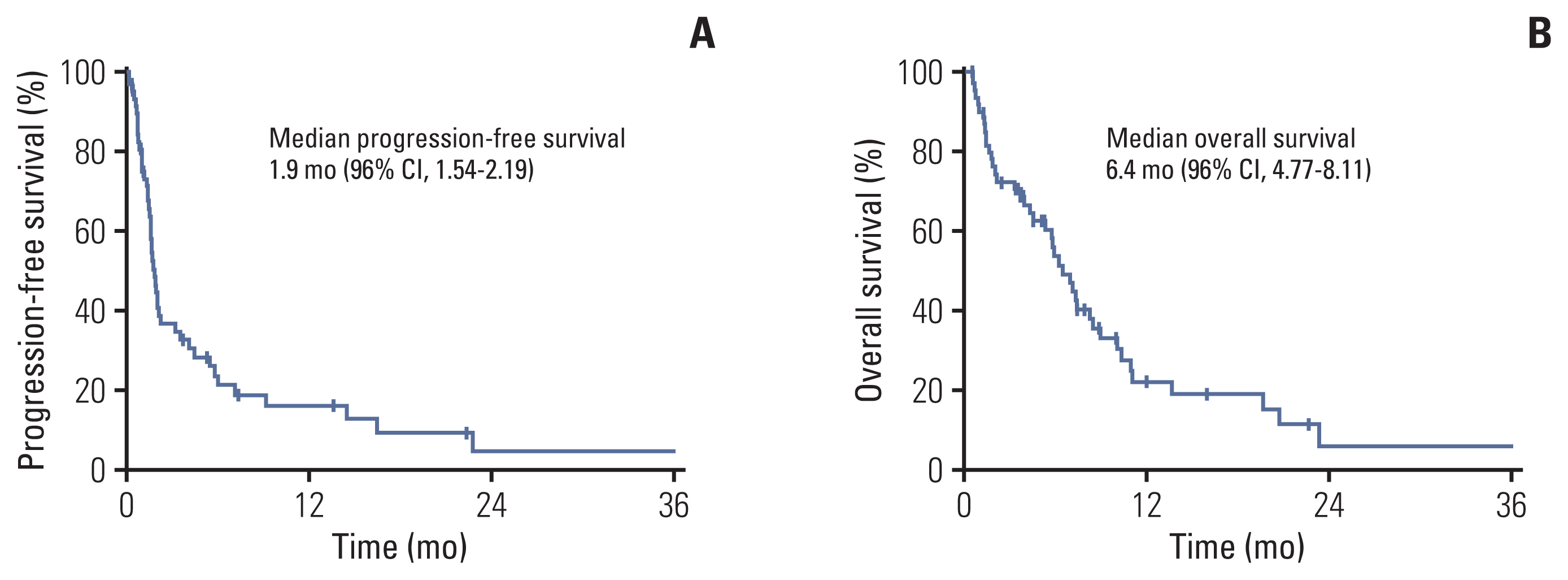
The median age of the patients was 68 years (range, 52 to 76 years), and 93.3% were male. Most patients had metastatic disease (81.7%) and had been previously treated with fluoropyrimidines, platinum, and taxane. In 53 patients with measurable disease, the overall response rate and disease control rate were 15.1% and 35.8%, respectively. With a median follow-up duration of 16.0 months, the median progression-free survival (PFS) and overall survival (OS) were 1.9 months (95% confidence interval [CI], 1.54 to 2.19) and 6.4 months (95% CI, 4.77 to 8.11), respectively. After multivariate analysis, recent use of antibiotics, low prognostic nutrition index (< 35.93), high Glasgow Prognosis Score (≥ 1) at baseline, and ≥ 1.4-fold increase in neutrophil-to-lymphocyte ratio after one cycle from baseline were significantly unfavorable factors for both PFS and OS. Younger age (< 65 years) was a significant factor for unfavorable PFS and hyponatremia (< 135 mmol/L) for unfavorable OS.
Conclusion
The use of ICIs after the failure of chemotherapy showed comparable efficacy in patients with advanced ESCC in real practice; this may be associated with host immune-nutritional status, which could be predicted by clinical and routine laboratory factors.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.
Article3. Shin A, Won YJ, Jung HK, Kong HJ, Jung KW, Oh CM, et al. Trends in incidence and survival of esophageal cancer in Korea: analysis of the Korea Central Cancer Registry Database. J Gastroenterol Hepatol. 2018; 33:1961–8.
Article4. Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008; 26:1435–42.
Article5. Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014; 15:78–86.
Article6. Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011; 47:2306–14.7. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018; 36:61–7.
Article8. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019; 5:546–50.
Article9. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017; 18:631–9.
Article10. Kojima T, Muro K, Francois E, Hsu CH, Moriwaki T, Kim SB, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J Clin Oncol. 2019; 37(2 4 Suppl):2.
Article11. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019; 20:1506–17.
Article12. Hall PS, Swinson D, Waters JS, Wadsley J, Falk S, Roy R, et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): the GO2 phase III trial. J Clin Oncol. 2019; 37(15 Suppl):4006.
Article13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article14. Chen MF, Chen PT, Kuan FC, Chen WC. The predictive value of pretreatment neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. Ann Surg Oncol. 2019; 26:190–9.
Article15. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984; 85:1001–5.16. Wang Y, Li P, Li J, Lai Y, Zhou K, Wang X, et al. The prognostic value of pretreatment Glasgow Prognostic Score in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. 2019; 11:8181–90.17. Feng JF, Wang L, Yang X, Chen S. Gustave Roussy Immune Score (GRIm-Score) is a prognostic marker in patients with resectable esophageal squamous cell carcinoma. J Cancer. 2020; 11:1334–40.
Article18. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018; 4:351–7.
Article19. Zingg U, Forberger J, Rajcic B, Langton C, Jamieson GG. Association of C-reactive protein levels and long-term survival after neoadjuvant therapy and esophagectomy for esophageal cancer. J Gastrointest Surg. 2010; 14:462–9.
Article20. Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, et al. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol. 2017; 22:1042–9.
Article21. Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017; 8:103117–28.
Article22. Li P, Wang X, Lai Y, Zhou K, Tang Y, Che G. The prognostic value of pre-treatment prognostic nutritional index in esophageal squamous cell carcinoma: a meta-analysis. Medicine (Baltimore). 2019; 98:e15280.23. Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018; 13:97–105.
Article24. Kurosaki T, Kawakami H, Mitani S, Kawabata R, Takahama T, Nonagase Y, et al. Glasgow Prognostic Score (GPS) and tumor response as biomarkers of nivolumab monotherapy in third- or later-line setting for advanced gastric cancer. In Vivo. 2020; 34:1921–9.
Article25. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020; 69:1813–22.
Article26. Moller M, Turzer S, Schutte W, Seliger B, Riemann D. Blood immune cell biomarkers in patient with lung cancer undergoing treatment with checkpoint blockade. J Immunother. 2020; 43:57–66.
Article27. Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019; 145:2541–6.
Article28. Seith F, Forschner A, Weide B, Guckel B, Schwartz M, Schwenck J, et al. Is there a link between very early changes of primary and secondary lymphoid organs in (18)F-FDG-PET/MRI and treatment response to checkpoint inhibitor therapy? J Immunother Cancer. 2020; 8:e000656.29. Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018; 11:47.
Article30. Gong J, Chehrazi-Raffle A, Placencio-Hickok V, Guan M, Hendifar A, Salgia R. The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin Transl Med. 2019; 8:9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors
- Advances in immune checkpoint inhibitors for hepatocellular carcinoma
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Current status of cancer immunotherapy with immune checkpoint inhibitors
- Gastrointestinal cancer treatment with immune checkpoint inhibitors