Cancer Res Treat.
2022 Apr;54(2):469-477. 10.4143/crt.2021.205.
Comparison of the Effectiveness and Clinical Outcome of Everolimus Followed by CDK4/6 Inhibitors with the Opposite Treatment Sequence in Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2528216
- DOI: http://doi.org/10.4143/crt.2021.205
Abstract
- Purpose
In hormone receptor-positive, human epidermal growth factor receptor 2–negative metastatic breast cancer (HR+ HER2–MBC), the mainstay treatment options include cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) and everolimus (EVE) in combination with endocrine treatment. This study aims to compare the outcomes of the following treatment sequences: CDK4/6i followed by EVE and EVE followed by CDK4/6i.
Materials and Methods
Data from HR+ HER2– MBC patients treated between January 2014 and November 2020 with both CDK4/6i and EVE were retrospectively analyzed.
Results
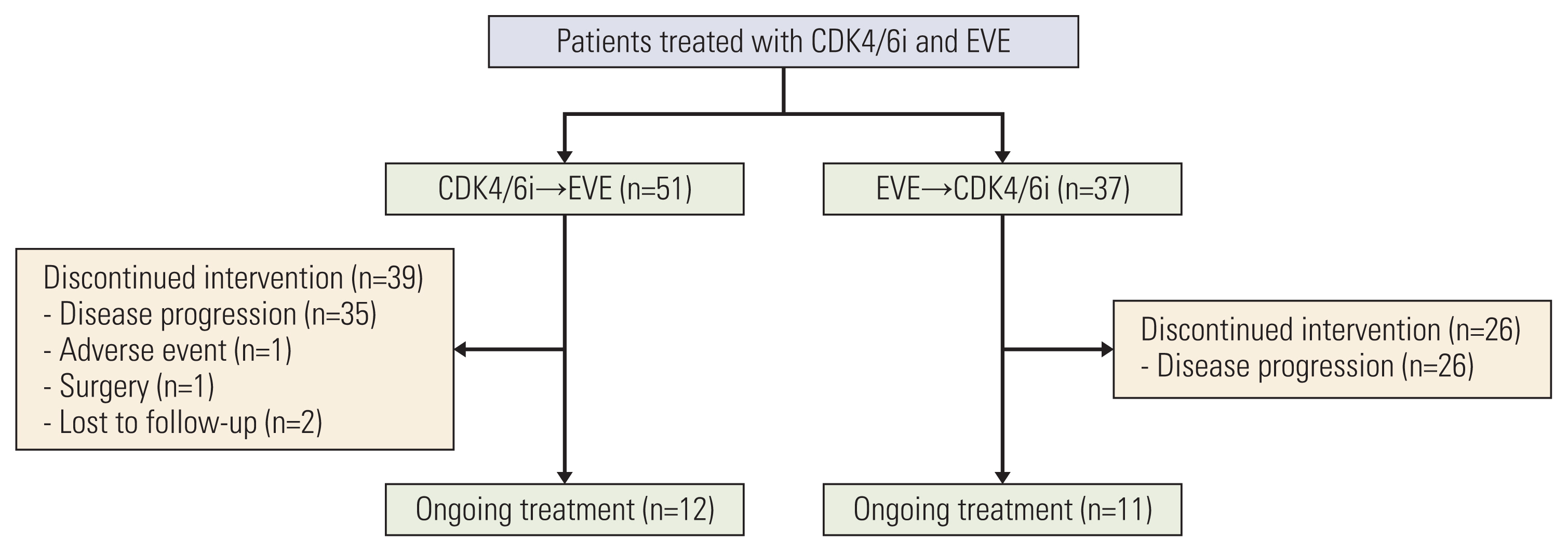
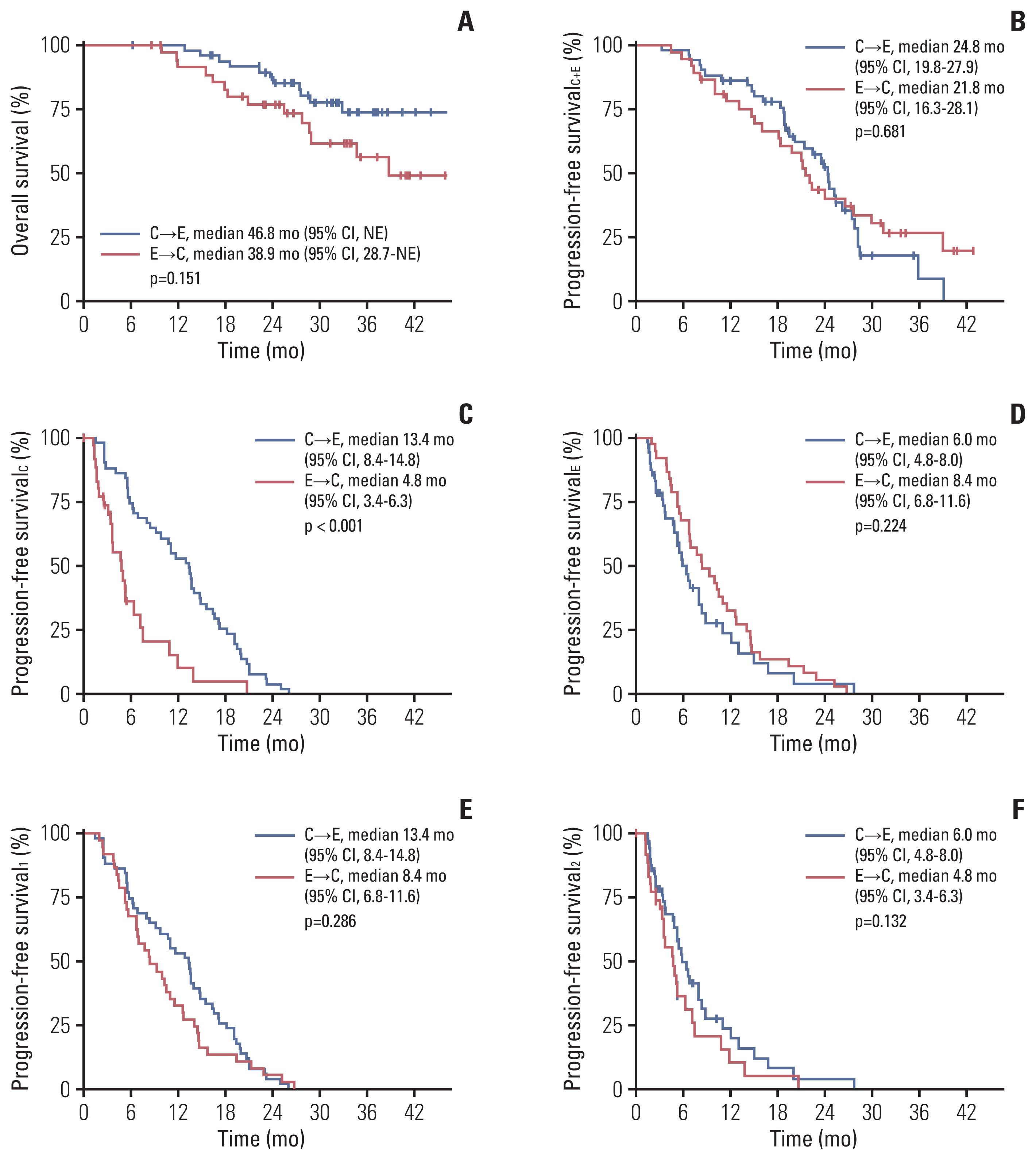
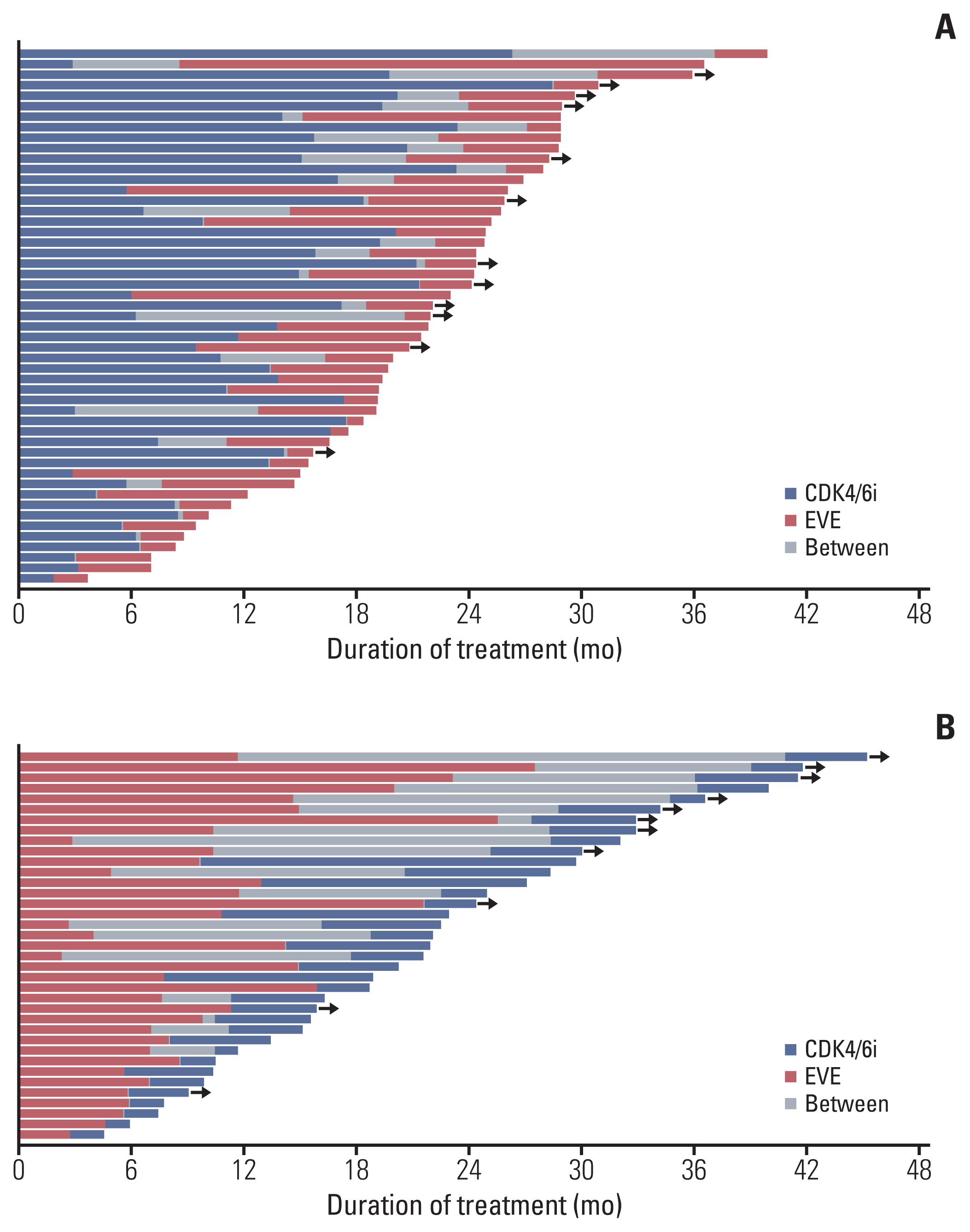
Among the 88 patients included in the study, 51 received CDK4/6i before EVE (C→E group), and 37 received EVE before CDK4/6i (E→C group) with endocrine treatment. More patients in the E→C group had endocrine resistance (13.7% vs. 40.5%), experienced palliative chemotherapy (7.8% vs. 40.5%), and were heavily treated (treated as ≥ 3rd line, 5.9% vs. 40.5%). Median overall survival was 46.8 months in the C→E group and 38.9 months in the E→C group (p=0.151). Median composite progression-free survival (PFS), defined as the time from the start of the preceding regimen to disease progression on the following regimen or death, was 24.8 months in the C→E group vs. 21.8 months in the E→C group (p=0.681). Median PFS2/PFS1 ratio did not differ significantly between groups (0.5 in the C→E group, 0.6 in the E→C group; p=0.775). Ten patients (11.4%) discontinued EVE, and two patients (2.3%) discontinued CDK4/6i during treatment.
Conclusion
Although the CDK4/6i-based regimen should be considered as an earlier line of treatment, CDK4/6i- and EVE-based treatments can be valid options in circumstances where the other treatment had been already given.
Keyword
Figure
Reference
-
References
1. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375:1925–36.
Article2. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017; 35:3638–46.
Article3. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016; 375:1738–48.
Article4. Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015; 373:209–19.
Article5. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017; 35:2875–84.
Article6. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018; 36:2465–72.
Article7. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018; 19:904–15.
Article8. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020; 6:116–24.
Article9. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019; 381:307–16.
Article10. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020; 382:514–24.
Article11. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012; 366:520–9.
Article12. Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 2014; 25:2357–62.13. Jansen VM, Bhola NE, Bauer JA, Formisano L, Lee KM, Hutchinson KE, et al. Kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res. 2017; 77:2488–99.
Article14. Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016; 76:2301–13.
Article15. Yamamoto T, Kanaya N, Somlo G, Chen S. Synergistic anticancer activity of CDK4/6 inhibitor palbociclib and dual mTOR kinase inhibitor MLN0128 in pRb-expressing ER-negative breast cancer. Breast Cancer Res Treat. 2019; 174:615–25.
Article16. Michaloglou C, Crafter C, Siersbaek R, Delpuech O, Curwen JO, Carnevalli LS, et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor-positive breast cancer. Mol Cancer Ther. 2018; 17:908–20.
Article17. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17:425–39.
Article18. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020; 31:1623–49.19. Chirila C, Mitra D, Colosia A, Ling C, Odom D, Iyer S, et al. Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: network meta-analysis. Curr Med Res Opin. 2017; 33:1457–66.
Article20. Cook MM, Al Rabadi L, Kaempf AJ, Saraceni MM, Savin MA, Mitri ZI. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021; 26:101–6.
Article21. Dhakal A, Antony Thomas R, Levine EG, Brufsky A, Takabe K, Hanna MG, et al. Outcome of everolimus-based therapy in hormone-receptor-positive metastatic breast cancer patients after progression on palbociclib. Breast Cancer (Auckl). 2020; 14:1178223420944864.
Article22. Dhakal A, Matthews CM, Levine EG, Salerno KE, Zhang F, Takabe K, et al. Efficacy of palbociclib combinations in hormone receptor-positive metastatic breast cancer patients after prior rverolimus yreatment. Clin Breast Cancer. 2018; 18:e1401–5.23. du Rusquec P, Palpacuer C, Campion L, Patsouris A, Augereau P, Gourmelon C, et al. Efficacy of palbociclib plus fulvestrant after everolimus in hormone receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2018; 168:559–66.
Article24. Kovac A, Kuhar CG, Ovcaricek T, Matos E, Mencinger M, Borstnar S. Efficacy of palbociclib after everolimus in hormone receptor-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2020; 38(15_Suppl):e13030.
Article25. O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018; 8:1390–403.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hormone Treatment for Breast Cancer
- Personalized therapy for advanced breast cancer using molecular signatures
- Comparison of Endocrine Therapies in Hormone Receptor-Positive and Human Epidermal Growth Factor Receptor 2-Negative Locally Advanced or Metastatic Breast Cancer: A Network Meta-Analysis
- Biomarkers of Everolimus Sensitivity in Hormone Receptor-Positive Breast Cancer
- Comparison of the Efficacy between First-Line Treatment Regimens for Patients with Hormone Receptor-Positive and HER2-Negative Metastatic Breast Cancer