J Korean Med Sci.
2022 Apr;37(14):e107. 10.3346/jkms.2022.37.e107.
The Risk of Tuberculosis in Patients With Inflammatory Bowel Disease Treated With Vedolizumab or Ustekinumab in Korea
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Mokdong Hospital, College of Medicine, Ewha Womans University, Seoul, Korea
- 3Department of Gastroenterology and Inflammatory Bowel Disease Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2528139
- DOI: http://doi.org/10.3346/jkms.2022.37.e107
Abstract
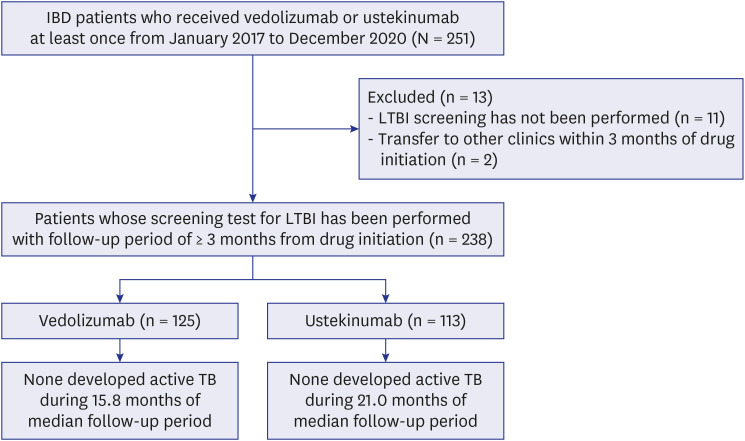
- The present study investigated the risk of active tuberculosis in patients with inflammatory bowel disease (IBD) treated with vedolizumab or ustekinumab, in actual clinical settings in a country with an intermediate tuberculosis burden. The medical records of 238 patients with IBD who received vedolizumab or ustekinumab were retrospectively reviewed at a tertiary referral center in South Korea. All patients had ≥ 3 months of follow-up duration and underwent a latent tuberculosis infection screening test before initiation of the administration of these drugs. Of the 238 patients enrolled, 181 had Crohn’s disease, and 57 had ulcerative colitis. During the median 18.7 months of follow-up, active tuberculosis did not develop in any patient treated with vedolizumab or ustekinumab. Therefore, we concluded that the risk of tuberculosis appears to be low in patients with IBD treated with vedolizumab or ustekinumab in South Korea.
Figure
Cited by 1 articles
-
Vedolizumab Is Safe and Efficacious for the Treatment of Pediatric-Onset Inflammatory Bowel Disease Patients Who Fail a Primary Biologic Agent
Sujin Choi, Eun Sil Kim, Yiyoung Kwon, Mi Jin Kim, Yon Ho Choe, Byung-Ho Choe, Ben Kang
J Korean Med Sci. 2022;37(37):e282. doi: 10.3346/jkms.2022.37.e282.
Reference
-
1. Shim TS. Diagnosis and treatment of latent tuberculosis infection due to initiation of anti-TNF therapy. Tuberc Respir Dis (Seoul). 2014; 76(6):261–268. PMID: 25024719.
Article2. Pagnini C, Pizarro TT, Cominelli F. Novel pharmacological therapy in inflammatory bowel diseases: beyond anti-tumor necrosis factor. Front Pharmacol. 2019; 10:671. PMID: 31316377.
Article3. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017; 14(5):269–278. PMID: 28144028.
Article4. Dobler CC. Biologic agents and tuberculosis. Microbiol Spectr. 2016; 4(6):4.6.49.
Article5. Ooi CJ, Hilmi IN, Kim HJ, Jalihal U, Wu DC, Demuth D, et al. Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study. Intest Res. 2021; 19(1):71–82. PMID: 32877600.
Article6. Banerjee R, Chuah SW, Hilmi IN, Wu DC, Yang SK, Demuth D, et al. Efficacy and safety of vedolizumab in Crohn’s disease in patients from Asian countries in the GEMINI 2 study. Intest Res. 2021; 19(1):83–94. PMID: 33378612.
Article7. Shin SY, Park SJ, Kim Y, Im JP, Kim HJ, Lee KM, et al. Clinical outcomes and predictors of response for adalimumab in patients with moderately to severely active ulcerative colitis: a KASID prospective multicenter cohort study. Intest Res. Forthcoming. 2021; DOI: 10.5217/ir.2021.00049.
Article8. Hisamatsu T, Kim HJ, Motoya S, Suzuki Y, Ohnishi Y, Fujii N, et al. Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI). Intest Res. 2021; 19(4):386–397. PMID: 33249802.
Article9. Korea Disease Control and Prevention Agency. Annual report on the notified tuberculosis in Korea 2019. Updated May 1, 2020. Accessed August 12, 2021. http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=2088 .10. Alrajhi S, Germain P, Martel M, Lakatos P, Bessissow T, Al-Taweel T, et al. Concordance between tuberculin skin test and interferon-gamma release assay for latent tuberculosis screening in inflammatory bowel disease. Intest Res. 2020; 18(3):306–314. PMID: 32182640.
Article11. Kang J, Jeong DH, Han M, Yang SK, Byeon JS, Ye BD, et al. Incidence of active tuberculosis within one year after tumor necrosis factor inhibitor treatment according to latent tuberculosis infection status in patients with inflammatory bowel disease. J Korean Med Sci. 2018; 33(47):e292. PMID: 30450023.
Article12. Kedia S, Mouli VP, Kamat N, Sankar J, Ananthakrishnan A, Makharia G, et al. Risk of tuberculosis in patients with inflammatory bowel disease on infliximab or adalimumab is dependent on the local disease burden of tuberculosis: a systematic review and meta-analysis. Am J Gastroenterol. 2020; 115(3):340–349. PMID: 32032073.
Article13. Lee JY, Oh K, Hong HS, Kim K, Hong SW, Park JH, et al. Risk and characteristics of tuberculosis after anti-tumor necrosis factor therapy for inflammatory bowel disease: a hospital-based cohort study from Korea. BMC Gastroenterol. 2021; 21(1):390. PMID: 34670529.
Article14. Ng SC, Hilmi IN, Blake A, Bhayat F, Adsul S, Khan QR, et al. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis. 2018; 24(11):2431–2441. PMID: 30312414.
Article15. Sandborn WJ, Rutgeerts P, Gasink C, Jacobstein D, Zou B, Johanns J, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther. 2018; 48(1):65–77. PMID: 29797519.
Article16. Hong SN, Kim HJ, Kim KH, Han SJ, Ahn IM, Ahn HS. Risk of incident Mycobacterium tuberculosis infection in patients with inflammatory bowel disease: a nationwide population-based study in South Korea. Aliment Pharmacol Ther. 2017; 45(2):253–263. PMID: 27933686.
Article17. Jung SM, Ju JH, Park MS, Kwok SK, Park KS, Kim HY, et al. Risk of tuberculosis in patients treated with anti-tumor necrosis factor therapy: a nationwide study in South Korea, a country with an intermediate tuberculosis burden. Int J Rheum Dis. 2015; 18(3):323–330. PMID: 25557144.
Article18. Yoo JW, Jo KW, Kang BH, Kim MY, Yoo B, Lee CK, et al. Mycobacterial diseases developed during anti-tumour necrosis factor-α therapy. Eur Respir J. 2014; 44(5):1289–1295. PMID: 25102962.
Article19. Bressler B, Yarur A, Silverberg MS, Bassel M, Bellaguarda E, Fourment C, et al. Vedolizumab and anti-tumour necrosis factor α real-world outcomes in biologic-naïve inflammatory bowel disease patients: results from the EVOLVE study. J Crohn’s Colitis. 2021; 15(10):1694–1706. PMID: 33786600.
Article20. Sands BE, Irving PM, Hoops T, Izanec JL, Gao LL, Gasink C, et al. 775d ustekinumab versus adalimumab for induction and maintenance therapy in moderate-to-severe Crohn’s disease: the SEAVUE study. Gastroenterology. 2021; 161(2):e30–e31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies
- Emerging Therapies: What Are Promising in the Near Future?
- The Phase 3 Trials of Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease
- Vedolizumab does not increase perioperative surgical complications in patients with inflammatory bowel disease, cohort study
- Successful treatment with vedolizumab in an adolescent with Crohn disease who had developed active pulmonary tuberculosis while receiving infliximab