Acute Crit Care.
2022 Feb;37(1):71-83. 10.4266/acc.2021.01326.
Comparison of high-flow nasal oxygen therapy and noninvasive ventilation in COVID-19 patients: a systematic review and meta-analysis
- Affiliations
-
- 1School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, North Jakarta, Indonesia
- 2Department of Internal Medicine, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, North Jakarta, Indonesia
- 3Department of Anesthesiology and Critical Care, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, North Jakarta, Indonesia
- KMID: 2527909
- DOI: http://doi.org/10.4266/acc.2021.01326
Abstract
- Background
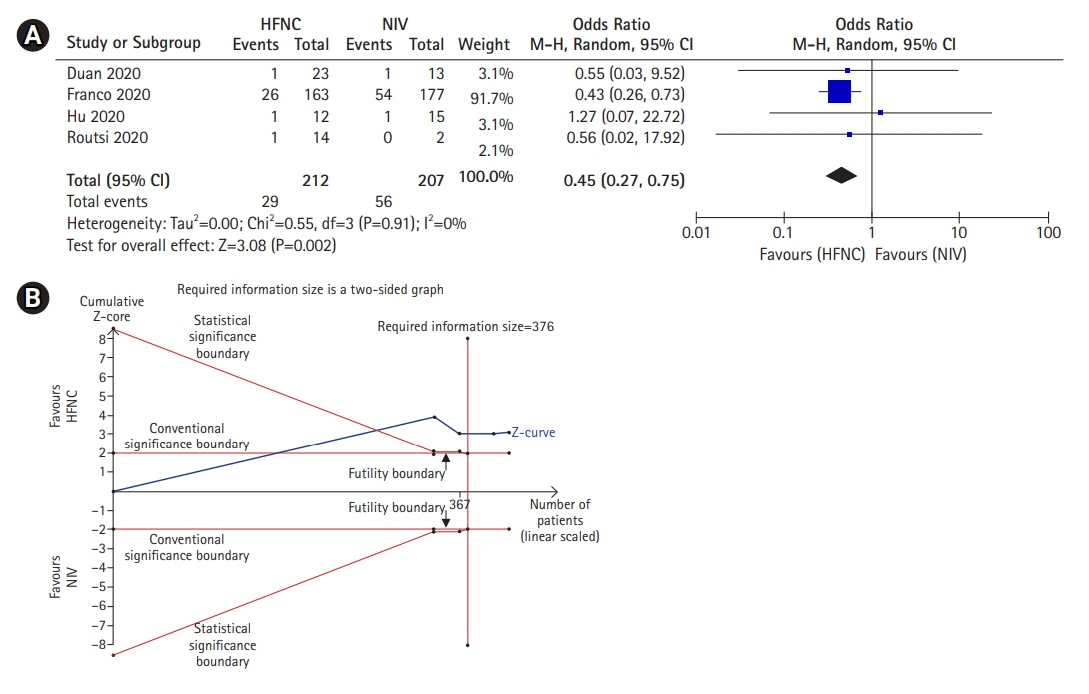
Acute respiratory failure (ARF) is a major adverse event commonly encountered in severe coronavirus disease 2019 (COVID-19). Although noninvasive mechanical ventilation (NIV) has long been used in the management of ARF, it has several adverse events which may cause patient discomfort and lead to treatment complication. Recently, high-flow nasal cannula (HFNC) has the potential to be an alternative for NIV in adults with ARF, including COVID-19 patients. The objective was to investigate the efficacy of HFNC compared to NIV in COVID-19 patients. Methods: This meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. Literature search was carried out in electronic databases for relevant articles published prior to June 2021. The protocol used in this study has been registered in International Prospective Register of Systematic Reviews (CRD42020225186). Results: Although the success rate of NIV is higher compared to HFNC (odds ratio [OR], 0.39; 95% confidence interval [CI], 0.16–0.97; P=0.04), this study showed that the mortality in the NIV group is also significantly higher compared to HFNC group (OR, 0.49; 95% CI, 0.39–0.63; P<0.001). Moreover, this study also demonstrated that there was no significant difference in intubation rates between the two groups (OR, 1.35; 95% CI, 0.86–2.11; P=0.19). Conclusions: Patients treated with HFNC showed better outcomes compared to NIV for ARF due to COVID-19. Therefore, HFNC should be considered prior to NIV in COVID-19–associated ARF. However, further studies with larger sample sizes are still needed to better elucidate the benefit of HFNC in COVID-19 patients.
Keyword
Figure
Reference
-
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA. 2020; 323:1239–42.
Article2. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180:934–43.
Article3. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019; 5:18.
Article4. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014; 370:980.
Article5. García-de-Acilu M, Patel BK, Roca O. Noninvasive approach for de novo acute hypoxemic respiratory failure: noninvasive ventilation, high-flow nasal cannula, both or none? Curr Opin Crit Care. 2019; 25:54–62.6. Crimi C, Noto A, Princi P, Esquinas A, Nava S. A European survey of noninvasive ventilation practices. Eur Respir J. 2010; 36:362–9.
Article7. Huang Y, Lei W, Zhang W, Huang JA. High-flow nasal cannula in hypercapnic respiratory failure: a systematic review and meta-analysis. Can Respir J. 2020; 2020:7406457.
Article8. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015; 372:2185–96.
Article9. Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020; 8:506–17.
Article10. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020; 46:854–87.
Article11. Duan J, Chen B, Liu X, Shu W, Zhao W, Li J, et al. Use of high-flow nasal cannula and noninvasive ventilation in patients with COVID-19: A multicenter observational study. Am J Emerg Med. 2021; 46:276–81.
Article12. Franco C, Facciolongo N, Tonelli R, Dongilli R, Vianello A, Pisani L, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020; 56:2002130.
Article13. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020; 10:37.
Article14. Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017; 189:E260–7.
Article15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article16. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.
Article17. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON): Ottawa Hospital Research Institute;2013. [cited 2021 Dec 10]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.18. Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA) [Internet]. Copenhagen: Copenhagen Trial Unit, Centre for Clinical Intervention Research;2011. [cited 2021 Dec 10]. Available from: https://ctu.dk/wp-content/uploads/2021/03/2017-10-10-TSA-Manual-ENG_ER.pdf.19. Cochrane Collaboration. Review Manager (RevMan) version 5.4. London: Cochrane Collaboration;2020.20. Hu HT, Xu S, Wang J, Rao X. Respiratory support in severely or critically ill ICU patients with COVID-19 in Wuhan, China. Curr Med Sci. 2020; 40:636–41.
Article21. Wang Z, Ye D, Wang M, Zhao M, Li D, Ye J, et al. Clinical features of COVID-19 patients with different outcomes in wuhan: a retrospective observational study. Biomed Res Int. 2020; 2020:2138387.
Article22. Wendel Garcia PD, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, Roche-Campo F, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020; 25:100449.23. COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021; 47:60–73.24. Klein SJ, Bellmann R, Dejaco H, Eschertzhuber S, Fries D, Furtwängler W, et al. Structured ICU resource management in a pandemic is associated with favorable outcome in critically ill COVID‑19 patients. Wien Klin Wochenschr. 2020; 132:653–63.
Article25. Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of Helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021; 325:1731–43.26. Routsi C, Magira E, Kokkoris S, Siembos I, Vrettou C, Zervakis D, et al. Hospital resources may be an important aspect of mortality rate among critically ill patients with COVID-19: the paradigm of Greece. J Clin Med. 2020; 9:3730.
Article27. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307:2526–33.28. Wendel Garcia PD, Aguirre-Bermeo H, Buehler PK, Alfaro-Farias M, Yuen B, David S, et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care. 2021; 25:175.
Article29. Koga Y, Kaneda K, Fujii N, Tanaka R, Miyauchi T, Fujita M, et al. Comparison of high-flow nasal cannula oxygen therapy and non-invasive ventilation as first-line therapy in respiratory failure: a multicenter retrospective study. Acute Med Surg. 2019; 7:e461.
Article30. Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016; 44:282–90.31. Grieco DL, Menga LS, Raggi V, Bongiovanni F, Anzellotti GM, Tanzarella ES, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020; 201:303–12.
Article32. Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011; 37:1780–6.
Article33. Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013; 58:1621–4.
Article34. Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017; 195:1207–15.
Article35. Esquinas Rodriguez AM, Scala R, Soroksky A, BaHammam A, de Klerk A, Valipour A, et al. Clinical review: humidifiers during non-invasive ventilation: key topics and practical implications. Crit Care. 2012; 16:203.
Article36. Placidi G, Cornacchia M, Polese G, Zanolla L, Assael BM, Braggion C. Chest physiotherapy with positive airway pressure: a pilot study of short-term effects on sputum clearance in patients with cystic fibrosis and severe airway obstruction. Respir Care. 2006; 51:1145–53.37. Lewis SR, Baker PE, Parker R, Smith AF. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev. 2021; 3:CD010172.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-flow nasal cannula oxygen therapy in children: a clinical review
- The Prevalence of Post-Traumatic Stress Disorder in the General Population during the COVID-19 Pandemic: A Systematic Review and Single-Arm Meta-Analysis
- Successful noninvasive ventilation in a severely acidotic and hypercapnic comatose COVID-19 patient with multiple comorbidities: a case report
- Clinical and Laboratory Features of Pediatric Patients with COVID-19: Systematic Review and Meta-analysis
- Predictors of Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis