Anat Cell Biol.
2022 Mar;55(1):63-71. 10.5115/acb.21.137.
Frequency, shape, and estimated volume of intracranial physiologic calcification in different age groups investigated by brain computed tomography scan: a retrospective study
- Affiliations
-
- 1Student Research Committee, Iran University of Medical Sciences, Tehran, Iran
- 2Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3Department of Anatomy, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- 4Reproductive Sciences and Technology Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- KMID: 2527670
- DOI: http://doi.org/10.5115/acb.21.137
Abstract
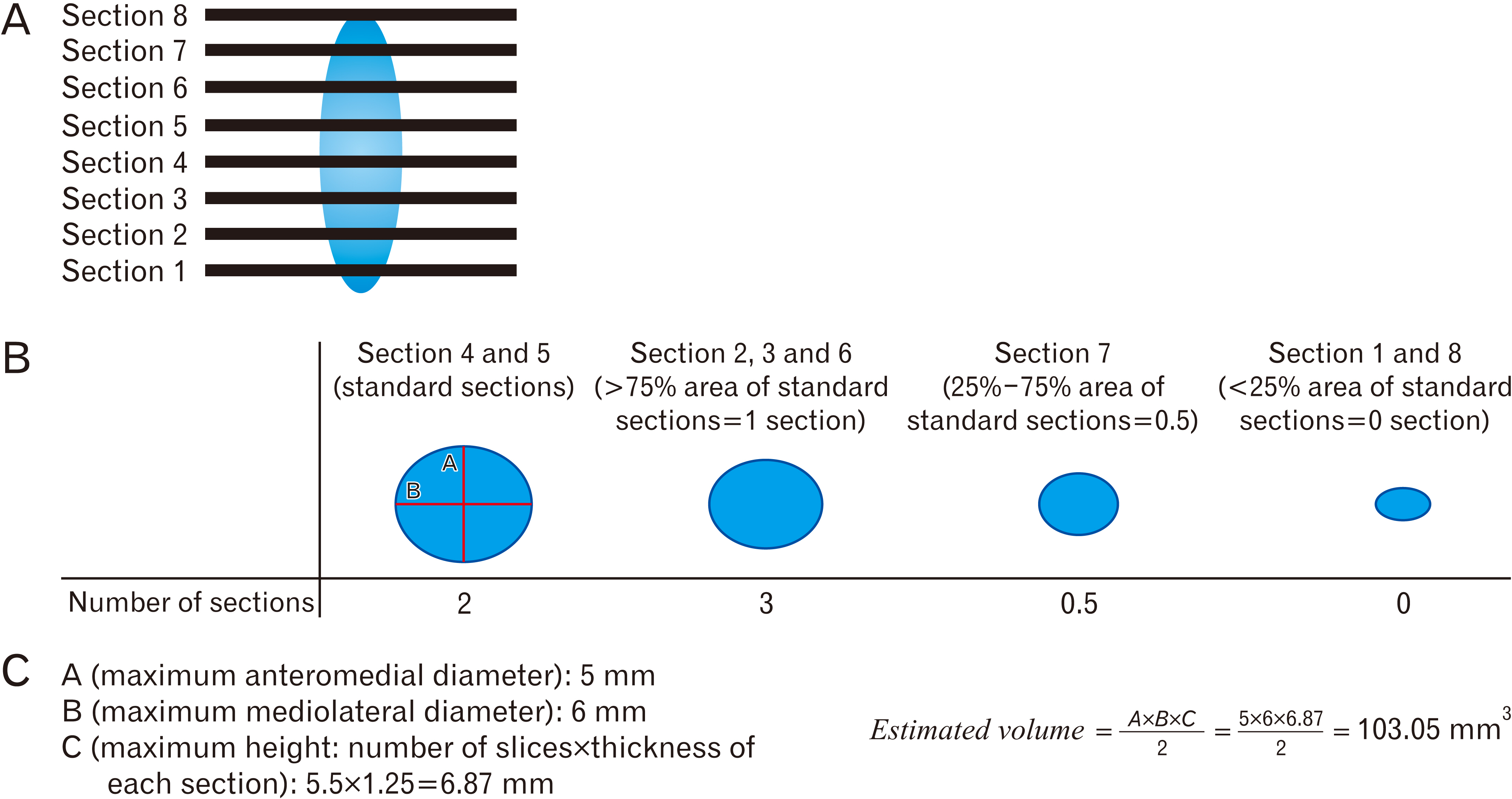
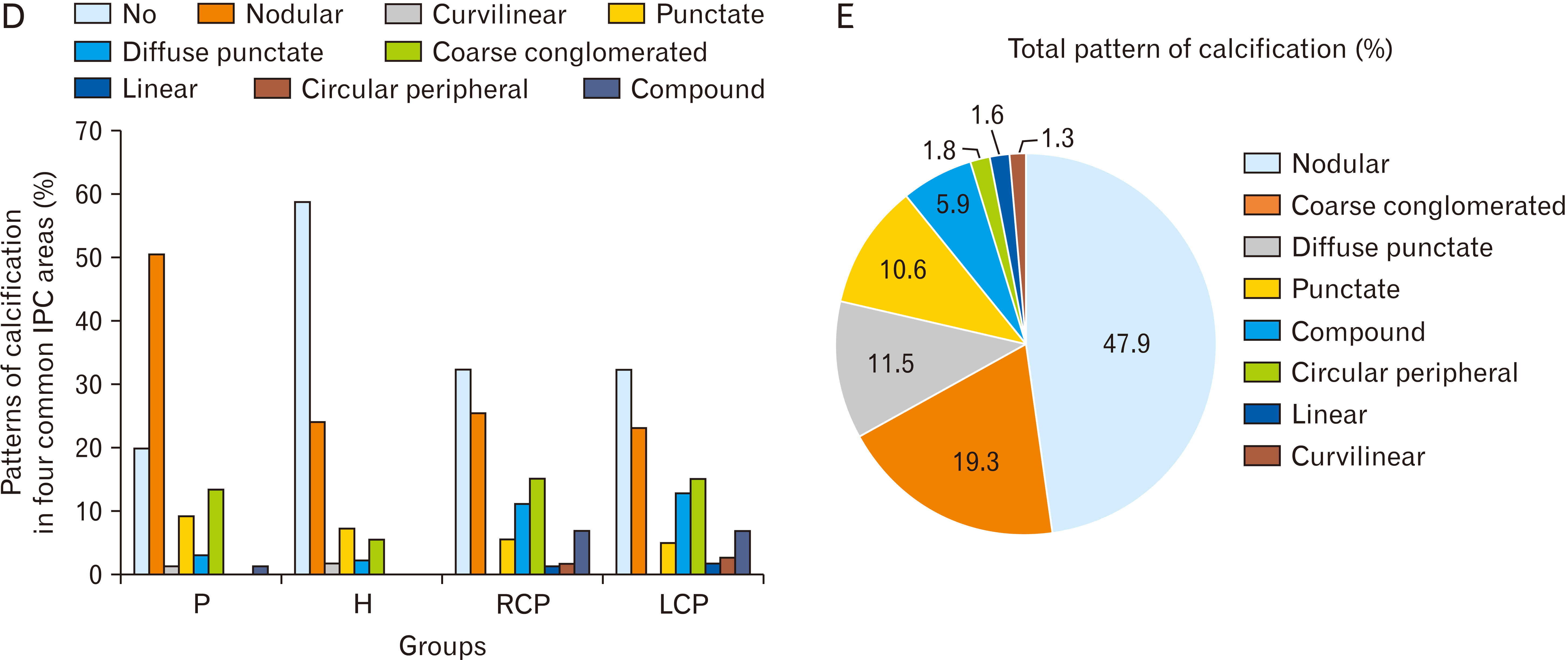
- Intracranial calcification is referred to calcification of parenchyma and vascular structures in brain which can be physiologic or pathologic. This study was conducted with the purpose of investigating the frequency, location, pattern, dimensions and estimated volume of intracranial physiologic calcification (IPC) by computer tomography in different age groups. In this cross-sectional retrospective study, brain computed tomography scans of 216 patients were analyzed in 9 age groups each containing 24 patients from 2 to 89 years old. Data were analyzed by SPSS software using one way analysis of variance (ANOVA, post hoc Tukey), chi square, and linear regression tests (P≤0.05 was considered significant). Rate of calcification in different areas were as follows: pineal gland (75.0%), habenula (36.4%), pineohabenula (15.0%), right lateral ventricle choroid plexus (RCP) (67.7%), left lateral ventricle choroid plexus (LCP) (62.7%), falx cerebri (26.8%), petroclinoid ligament (13.2%), tentorium cerebelli (6.8%), third ventricle choroid plexus (0.9%), fourth ventricle choroid plexus (2.7%), basal ganglia (0.9%). A significant correlation exists between the presence of calcification in pineal, habenula, RCP, and LCP (P≤0.001). Nodular shape of calcification was dominant (47.9%). Estimated volume of pineal calcification showed increased levels in group 8 (70–79 years old) compared to group 2 (10–19 years old) (P≤0.05). Since the accurate description of radiologic appearance of IPCs (location, shape, and size) accompanied with age and clinical manifestation is of great importance in diagnosis and distinguishing from pathologic calcification—for example in patients with melatonin dysregulation or schizophrenic patients—this study was required.
Keyword
Figure
Cited by 1 articles
-
In search of subcortical and cortical morphologic alterations of a normal brain through aging: an investigation by computed tomography scan
Mehrdad Ghorbanlou, Fatemeh Moradi, Mohammad Hassan Kazemi-Galougahi, Maasoume Abdollahi
Anat Cell Biol. 2024;57(1):45-60. doi: 10.5115/acb.23.219.
Reference
-
References
1. Saade C, Najem E, Asmar K, Salman R, El Achkar B, Naffaa L. 2019; Intracranial calcifications on CT: an updated review. J Radiol Case Rep. 13:1–18. DOI: 10.3941/jrcr.v13i8.3633. PMID: 31558966. PMCID: PMC6738489.2. Spector R, Keep RF, Robert Snodgrass S, Smith QR, Johanson CE. 2015; A balanced view of choroid plexus structure and function: focus on adult humans. Exp Neurol. 267:78–86. DOI: 10.1016/j.expneurol.2015.02.032. PMID: 25747036.
Article3. Møller M, Baeres FM. 2002; The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 309:139–50. DOI: 10.1007/s00441-002-0580-5. PMID: 12111544.
Article4. Boulos LJ, Darcq E, Kieffer BL. 2017; Translating the habenula-from rodents to humans. Biol Psychiatry. 81:296–305. DOI: 10.1016/j.biopsych.2016.06.003. PMID: 27527822. PMCID: PMC5143215.
Article5. Adeeb N, Mortazavi M, Tubbs RS, Cohen-Gadol A. 2012; The cranial dura mater: a review of its history, embryology, and anatomy. Childs Nerv Syst. 28:827–37. DOI: 10.1007/s00381-012-1744-6. PMID: 22526439.
Article6. Lanciego JL, Luquin N, Obeso JA. 2012; Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2:a009621. DOI: 10.1101/cshperspect.a009621. PMID: 23071379. PMCID: PMC3543080.
Article7. Pilli VK, Behen ME, Hu J, Xuan Y, Janisse J, Chugani HT, Juhász C. 2017; Clinical and metabolic correlates of cerebral calcifications in Sturge-Weber syndrome. Dev Med Child Neurol. 59:952–8. DOI: 10.1111/dmcn.13433. PMID: 28397986. PMCID: PMC5568960.
Article8. Dinizio A, Vincent J, Nickerson J. 2015; Intracranial calcifications in the pediatric age group: an imaging review. J Pediatr Neuroradiol. 4:49–59. DOI: 10.1055/s-0036-1583312.
Article9. Kim JM, Park KY, Bae JH, Han SH, Jeong HB, Jeong D. 2019; Intracranial arterial calcificationes can reflect cerebral atherosclerosis burden. J Clin Neurol. 15:38–45. DOI: 10.3988/jcn.2019.15.1.38. PMID: 30375758. PMCID: PMC6325360.
Article10. Grech R, Grech S, Mizzi A. 2012; Intracranial calcifications. A pictorial review. Neuroradiol J. 25:427–51. DOI: 10.1177/197140091202500406. PMID: 24029036.11. Kontzialis M, Huisman TAGM. 2018; Toxic-metabolic neurologic disorders in children: a neuroimaging review. J Neuroimaging. 28:587–95. DOI: 10.1111/jon.12551. PMID: 30066477.
Article12. Daghighi MH, Rezaei V, Zarrintan S, Pourfathi H. 2007; Intracranial physiological calcifications in adults on computed tomography in Tabriz, Iran. Folia Morphol (Warsz). 66:115–9.13. Kitkhuandee A, Sawanyawisuth K, Johns NP, Kanpittaya J, Johns J. 2014; Pineal calcification is associated with symptomatic cerebral infarction. J Stroke Cerebrovasc Dis. 23:249–53. DOI: 10.1016/j.jstrokecerebrovasdis.2013.01.009. PMID: 23434443.
Article14. Sedghizadeh PP, Nguyen M, Enciso R. 2012; Intracranial physiological calcifications evaluated with cone beam CT. Dentomaxillofac Radiol. 41:675–8. DOI: 10.1259/dmfr/33077422. PMID: 22842632. PMCID: PMC3528191.
Article15. Turgut AT, Karakaş HM, Ozsunar Y, Altın L, Ceken K, Alıcıoğlu B, Sönmez I, Alparslan A, Yürümez B, Celik T, Kazak E, Geyik PÖ, Koşar U. 2008; Age-related changes in the incidence of pineal gland calcification in Turkey: a prospective multicenter CT study. Pathophysiology. 15:41–8. DOI: 10.1016/j.pathophys.2008.02.001. PMID: 18420391.
Article16. Yalcin A, Ceylan M, Bayraktutan OF, Sonkaya AR, Yuce I. 2016; Age and gender related prevalence of intracranial calcifications in CT imaging; data from 12,000 healthy subjects. J Chem Neuroanat. 78:20–4. DOI: 10.1016/j.jchemneu.2016.07.008. PMID: 27475519.
Article17. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. 1996; The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 27:1304–5. DOI: 10.1161/01.STR.27.8.1304. PMID: 8711791.
Article18. Webb AJ, Ullman NL, Morgan TC, Muschelli J, Kornbluth J, Awad IA, Mayo S, Rosenblum M, Ziai W, Zuccarrello M, Aldrich F, John S, Harnof S, Lopez G, Broaddus WC, Wijman C, Vespa P, Bullock R, Haines SJ, Cruz-Flores S, Tuhrim S, Hill MD, Narayan R, Hanley DF. 2015; Accuracy of the ABC/2 score for intracerebral hemorrhage: systematic review and analysis of MISTIE, CLEAR-IVH, and CLEAR III. Stroke. 46:2470–6. DOI: 10.1161/STROKEAHA.114.007343. PMID: 26243227. PMCID: PMC4550520.
Article19. Won SY, Zagorcic A, Dubinski D, Quick-Weller J, Herrmann E, Seifert V, Konczalla J. 2018; Excellent accuracy of ABC/2 volume formula compared to computer-assisted volumetric analysis of subdural hematomas. PLoS One. 13:e0199809. DOI: 10.1371/journal.pone.0199809. PMID: 29944717. PMCID: PMC6019668.
Article20. Macpherson P, Matheson MS. 1979; Comparison of calcification of pineal, habenular commissure and choroid plexus on plain films and computed tomography. Neuroradiology. 18:67–72. DOI: 10.1007/BF00344824. PMID: 471224.
Article21. Ciavarella C, Gallitto E, Ricci F, Buzzi M, Stella A, Pasquinelli G. 2017; The crosstalk between vascular MSCs and inflammatory mediators determines the pro-calcific remodelling of human atherosclerotic aneurysm. Stem Cell Res Ther. 8:99. DOI: 10.1186/s13287-017-0554-x. PMID: 28446225. PMCID: PMC5406974.
Article22. Tan DX, Xu B, Zhou X, Reiter RJ. 2018; Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules. 23:301. DOI: 10.3390/molecules23020301. PMID: 29385085. PMCID: PMC6017004.
Article23. Kitkhuandee A, Sawanyawisuth K, Johns J, Kanpittaya J, Tuntapakul S, Johns NP. 2014; Pineal calcification is a novel risk factor for symptomatic intracerebral hemorrhage. Clin Neurol Neurosurg. 121:51–4. DOI: 10.1016/j.clineuro.2014.03.019. PMID: 24793475.
Article24. Sandyk R. 1992; Pineal and habenula calcification in schizophrenia. Int J Neurosci. 67:19–30. DOI: 10.3109/00207459208994773. PMID: 1305634.
Article25. Kwak R, Takeuchi F, Yamamoto N, Nakamura T, Kadoya S. 1988; Intracranial physiological calcification on computed tomography (Part 2): calcification in the choroid plexus of the lateral ventricles. No To Shinkei. 40:707–11. Japanese.26. Whitehead MT, Oh C, Raju A, Choudhri AF. 2015; Physiologic pineal region, choroid plexus, and dural calcifications in the first decade of life. AJNR Am J Neuroradiol. 36:575–80. DOI: 10.3174/ajnr.A4153. PMID: 25355815. PMCID: PMC8013049.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Idiopathic Hypoparathyroidism Combined with Extensive Intracranial Calcification: A Case Report
- Computed tomography of calcification of the basal ganglia
- Intracranial Calcification Caused by a Brain Abscess : A Rare Cause of Intracranial Calcification
- Basal Ganglia Calcification and Hypoparathyroidism: Case Report
- Intracranial Arterial Calcificationes Can Reflect Cerebral Atherosclerosis Burden