J Korean Med Sci.
2022 Mar;37(11):e87. 10.3346/jkms.2022.37.e87.
Volumetric Splenomegaly in Patients With Polycythemia Vera
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 2Department of Laboratory Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 3Department of Radiology, Chungnam National University College of Medicine, Daejeon, Korea
- KMID: 2527447
- DOI: http://doi.org/10.3346/jkms.2022.37.e87
Abstract
- Background
Non-palpable splenomegaly in patients with polycythemia vera (PV) has seldom been addressed. In this retrospective study, we evaluated non-palpable, volumetric splenomegaly defined based on age- and body surface area (BSA)–matched criteria in patients with PV diagnosed according to the 2016 World Health Organization diagnostic criteria.
Methods
Patients with PV who underwent abdominal computed tomography (CT) and who had palpable splenomegaly at diagnosis from January 1991 to December 2020 at Chungnam National University Hospital were enrolled. The spleen volume of each patient was determined by volumetric analysis of abdominal CT and adjusted for the patient’s age and BSA. Then the degree of splenomegaly was classified as no splenomegaly, borderline volumetric splenomegaly, overt volumetric splenomegaly, or palpable splenomegaly.
Results
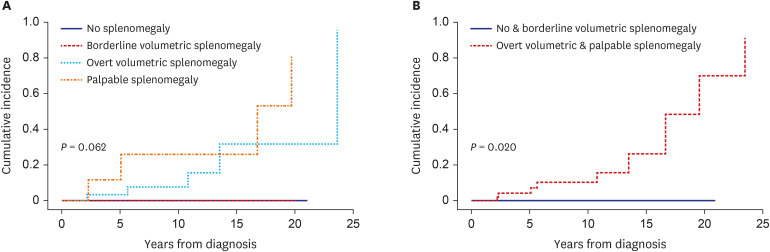
Of the 87 PV patients enrolled, 15 (17.2%) had no splenomegaly, whereas 17 (19.5%), 45 (51.7%), and 10 (11.5%) had borderline volumetric, overt volumetric, and palpable splenomegaly, respectively. The degree of splenomegaly did not affect the cumulative incidence of thrombotic vascular events (10-year incidence: 7.7%, 0%, 22.3%, and 50.7%, respectively, P = 0.414). By contrast, splenomegaly tended to adversely affect myelofibrotic transformation (10-year cumulative incidence: 0%, 0%, 7.1%, and 30.3%, respectively, P = 0.062). Moreover, the cumulative incidence of myelofibrotic transformation was significantly higher in patients with overt volumetric or palpable splenomegaly than those with no or borderline volumetric splenomegaly (10-year incidence: 0% vs. 10.3%, respectively; 15-year incidence: 0% vs. 26.3%, respectively, P = 0.020). Overall survival (OS) differed among patients with different degrees of splenomegaly (15-year OS: 100%, 78.6%, 71.7%, and 51.9%, respectively, P = 0.021).
Conclusion
The degree of splenomegaly, including volumetric splenomegaly, based on ageand BSA-matched reference spleen volumes at diagnosis reflects disease progression in PV patients. Therefore, volumetric splenomegaly should be evaluated at the time of diagnosis and taken into consideration when predicting the prognosis of patients with PV.
Figure
Reference
-
1. Spivak JL. Polycythemia vera. Curr Treat Options Oncol. 2018; 19(2):12. PMID: 29516275.2. Mesa R. Myeloproliferative disorder-associated massive splenomegaly. Clin Adv Hematol Oncol. 2008; 6(4):278. PMID: 18496494.3. Song MK, Park BB, Uhm JE. Understanding splenomegaly in myelofibrosis: association with molecular pathogenesis. Int J Mol Sci. 2018; 19(3):E898. PMID: 29562644.4. Hehlmann R, Jahn M, Baumann B, Köpcke W. Essential thrombocythemia. Clinical characteristics and course of 61 cases. Cancer. 1988; 61(12):2487–2496. PMID: 3365670.5. Jantunen R, Juvonen E, Ikkala E, Oksanen K, Anttila P, Hormila P, et al. Essential thrombocythemia at diagnosis: causes of diagnostic evaluation and presence of positive diagnostic findings. Ann Hematol. 1998; 77(3):101–106. PMID: 9797078.6. Chim CS, Kwong YL, Lie AK, Ma SK, Chan CC, Wong LG, et al. Long-term outcome of 231 patients with essential thrombocythemia: prognostic factors for thrombosis, bleeding, myelofibrosis, and leukemia. Arch Intern Med. 2005; 165(22):2651–2658. PMID: 16344424.7. Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004; 117(10):755–761. PMID: 15541325.8. Xin CH, Xu JQ, Sui JR, Wang XL. Analysis on 71 patients with polycythemia vera. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012; 20(3):667–670. PMID: 22739179.9. Michiels JJ, Juvonen E. Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group. Semin Thromb Hemost. 1997; 23(4):339–347. PMID: 9263350.10. Thiele J, Kvasnicka HM. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Curr Hematol Malig Rep. 2009; 4(1):33–40. PMID: 20425436.11. Carneskog J, Wadenvik H, Fjälling M, Kutti J. Assessment of spleen size using gamma camera scintigraphy in newly diagnosed patients with essential thrombocythaemia and polycythaemia vera. Eur J Haematol. 1996; 56(3):158–162. PMID: 8598235.12. Messinezy M, Macdonald LM, Nunan TO, Westwood NB, Chinn S, Pearson TC. Spleen sizing by ultrasound in polycythaemia and thrombocythaemia: comparison with SPECT. Br J Haematol. 1997; 98(1):103–107. PMID: 9233571.13. Picardi M, Martinelli V, Ciancia R, Soscia E, Morante R, Sodano A, et al. Measurement of spleen volume by ultrasound scanning in patients with thrombocytosis: a prospective study. Blood. 2002; 99(11):4228–4230. PMID: 12010832.14. Accurso V, Santoro M, Raso S, Contrino AD, Casimiro P, Piazza FD, et al. Splenomegaly impacts prognosis in essential thrombocythemia and polycythemia vera: a single center study. Hematol Rep. 2019; 11(4):8281. PMID: 31871612.15. Kaneko J, Sugawara Y, Matsui Y, Ohkubo T, Makuuchi M. Normal splenic volume in adults by computed tomography. Hepatogastroenterology. 2002; 49(48):1726–1727. PMID: 12397778.16. Caglar V, Alkoc OA, Uygur R, Serdaroglu O, Ozen OA. Determination of normal splenic volume in relation to age, gender and body habitus: a stereological study on computed tomography. Folia Morphol (Warsz). 2014; 73(3):331–338. PMID: 25465038.17. Harris A, Kamishima T, Hao HY, Kato F, Omatsu T, Onodera Y, et al. Splenic volume measurements on computed tomography utilizing automatically contouring software and its relationship with age, gender, and anthropometric parameters. Eur J Radiol. 2010; 75(1):e97–101.18. Sprogøe-Jakobsen S, Sprogøe-Jakobsen U. The weight of the normal spleen. Forensic Sci Int. 1997; 88(3):215–223. PMID: 9291593.19. Kirito K, Suzuki K, Miyamura K, Takeuchi M, Handa H, Okamoto S, et al. Ruxolitinib is effective and safe in Japanese patients with hydroxyurea-resistant or hydroxyurea-intolerant polycythemia vera with splenomegaly. Int J Hematol. 2018; 107(2):173–184. PMID: 28956263.20. Griesshammer M, Saydam G, Palandri F, Benevolo G, Egyed M, Callum J, et al. Ruxolitinib for the treatment of inadequately controlled polycythemia vera without splenomegaly: 80-week follow-up from the RESPONSE-2 trial. Ann Hematol. 2018; 97(9):1591–1600. PMID: 29804268.21. Song IC, Yeon SH, Lee MW, Ryu H, Lee HJ, Yun HJ, et al. Thrombotic and hemorrhagic events in 2016 World Health Organization-defined Philadelphia-negative myeloproliferative neoplasm. Korean J Intern Med. 2021; 36(5):1190–1203. PMID: 34289585.22. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127(20):2391–2405. PMID: 27069254.23. Lee MW, Yeon SH, Ryu H, Song IC, Lee HJ, Yun HJ, et al. Volumetric splenomegaly in patients with essential thrombocythemia and prefibrotic/early primary myelofibrosis. Int J Hematol. 2021; 114(1):35–43. PMID: 33704663.24. Cerquozzi S, Barraco D, Lasho T, Finke C, Hanson CA, Ketterling RP, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017; 7(12):662. PMID: 29282357.25. Bai J, Xue Y, Ye L, Yao J, Zhou C, Shao Z, et al. Risk factors of long-term incidences of thrombosis, myelofibrosis and evolution into malignance in polycythemia vera: a single center experience from China. Int J Hematol. 2008; 88(5):530–535. PMID: 19002391.26. Bai J, Ai L, Zhang L, Yang FC, Zhou Y, Xue Y. Incidence and risk factors for myelofibrotic transformation among 272 Chinese patients with JAK2-mutated polycythemia vera. Am J Hematol. 2015; 90(12):1116–1121. PMID: 26370613.27. Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015; 5(11):e366. PMID: 26565403.28. Mitra D, Kaye JA, Piecoro LT, Brown J, Reith K, Mughal TI, et al. Symptom burden and splenomegaly in patients with myelofibrosis in the United States: a retrospective medical record review. Cancer Med. 2013; 2(6):889–898. PMID: 24403262.29. Wang J, Xu J, Gale RP, Xu Z, Li B, Qin T, et al. Prognostic impact of splenomegaly on survival of Chinese with primary myelofibrosis. Leuk Res. 2014; 38(10):1207–1211. PMID: 25182689.30. Tefferi A, Strand JJ, Lasho TL, Knudson RA, Finke CM, Gangat N, et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia. 2007; 21(9):2074–2075. PMID: 17476276.31. Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007; 21(9):1952–1959. PMID: 17625606.32. Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010; 24(9):1574–1579. PMID: 20631743.33. Silver RT, Vandris K, Wang YL, Adriano F, Jones AV, Christos PJ, et al. JAK2(V617F) allele burden in polycythemia vera correlates with grade of myelofibrosis, but is not substantially affected by therapy. Leuk Res. 2011; 35(2):177–182. PMID: 20650526.34. Zhao S, Zhang X, Xu Y, Feng Y, Sheng W, Cen J, et al. Impact of JAK2V617F mutation burden on disease phenotype in Chinese patients with JAK2V617F-positive polycythemia vera (PV) and essential thrombocythemia (ET). Int J Med Sci. 2016; 13(1):85–91. PMID: 26917989.35. Kough RH. Idiopathic myelofibrosis with myeloid metaplasia of the spleen; a disease entity being recognized with increasing frequency. Med Times. 1966; 94(4):489–496. PMID: 5908056.36. Kirshner JJ, Goldberg J, Landaw SA. The spleen as a site of colony-forming cell production in myelofibrosis. Proc Soc Exp Biol Med. 1980; 165(2):279–282. PMID: 6934551.37. Douay L, Laporte JP, Lefrancois G, Najman A, Dupuy-Montbrun MC, Lopez M, et al. Blood and spleen haematopoiesis in patients with myelofibrosis. Leuk Res. 1987; 11(8):725–730. PMID: 3626614.38. Passamonti F, Mora B, Barraco D, Maffioli M. Post-ET and post-PV myelofibrosis: updates on a distinct prognosis from primary myelofibrosis. Curr Hematol Malig Rep. 2018; 13(3):173–182. PMID: 29713873.39. Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013; 27(9):1874–1881. PMID: 23739289.