Neonatal Med.
2022 Feb;29(1):46-54. 10.5385/nm.2022.29.1.46.
Is Less Invasive Surfactant Administration Better than INtubation-SURfactant-Extubation for Prophylactic Surfactant Replacement Therapy?
- Affiliations
-
- 1Department of Pediatrics, Inje University Sanggye Paik Hospital, Seoul, Korea
- KMID: 2527260
- DOI: http://doi.org/10.5385/nm.2022.29.1.46
Abstract
- Purpose
The study aimed to examine whether prophylactic surfactant replacement therapy (SRT) with less invasive surfactant administration (LISA) by tracheal catheterization in a group of spontaneously breathing preterm infants would improve clinical outcomes compared to prophylactic SRT with the INtubation-SURfactantExtubation (INSURE) method.
Methods
We compared 20 spontaneously breathing preterm infants, 25 to 29 weeks of gestation or with a birth weight of less than 1,250 g, treated with prophylactic SRT using a gastric tube (LISA group), to the 20 spontaneously breathing preterm infants matched by gestational age and birth weight, managed with prophylactic SRT via the INSURE method (INSURE group, historical control).
Results
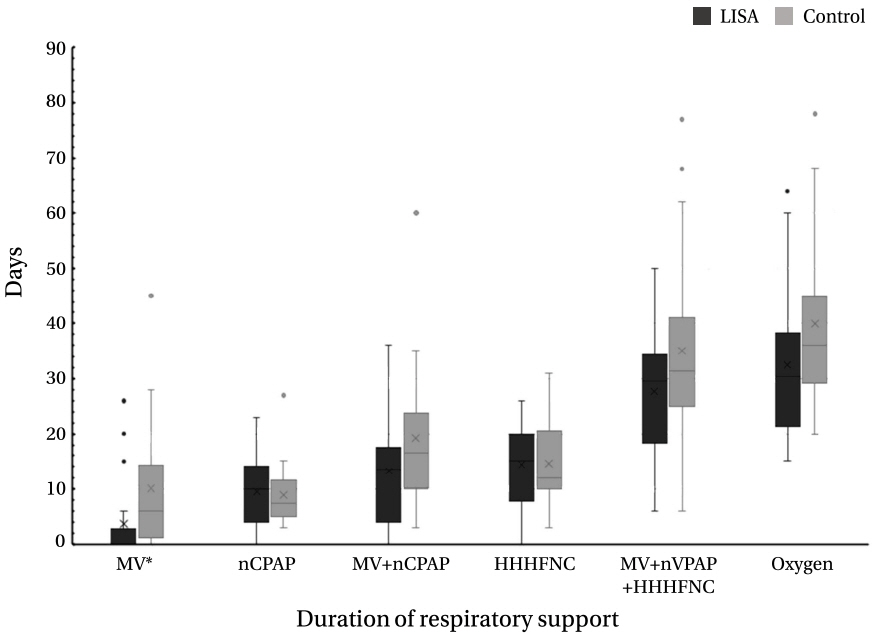
The LISA group had lower rates of mechanical ventilation (MV) 72 hours after birth (P=0.019) and at any time (P=0.025), lower frequency of bradycardia during SRT (P=0.031), and lower median duration of MV than the INSURE group (P=0.038). In multivariate analysis, the LISA method was associated with a significantly lower likelihood of receiving invasive ventilation during hospitalization (odds ratio [OR], 0.029; 95% confidence interval [CI], 0.001 to 0.938; P=0.046) and a decreased frequency of bradycardia during SRT (OR, 0.020; 95% CI, 0.001 to 0.535; P=0.020) as compared to the INSURE method.
Conclusion
Prophylactic SRT using LISA via tracheal catheterization in preterm infants may significantly reduce exposure to MV during hospitalization and bradycardia during surfactant administration.
Figure
Reference
-
1. Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002; 110(1 Pt 1):143–51.2. Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2009; 2:CD007836.3. Soll RF, Morley CJ. Prophylactic versus selective use of surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2000; 2:CD000510.4. Attar MA, Donn SM. Mechanisms of ventilator-induced lung injury in premature infants. Semin Neonatol. 2002; 7:353–60.5. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants: 2013 update. Neonatology. 2013; 103:353–68.6. Committee on Fetus and Newborn; American Academy of Pediatrics. Respiratory support in preterm infants at birth. Pediatrics. 2014; 133:171–4.7. Aguar M, Vento M, Dargaville PA. Minimally invasive surfactant therapy: an update. NeoReviews. 2014; 15:e275–85.8. Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med. 1994; 331:1051–5.9. Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics. 1999; 103:E24.10. Kribs A, Hartel C, Kattner E, Vochem M, Kuster H, Moller J, et al. Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klin Padiatr. 2010; 222:13–7.11. Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013; 131:e502–9.12. Rigo V, Lefebvre C, Broux I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur J Pediatr. 2016; 175:1933–42.13. Lau C, Chamberlain RS, Sun S. Less invasive surfactant administration reduces the need for mechanical ventilation in preterm infants: a meta-analysis. Glob Pediatr Health. 2017; 4:2333794X17696683.14. Gopel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an openlabel, randomised, controlled trial. Lancet. 2011; 378:1627–34.15. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987; 17:213–88.16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.17. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123:991–9.18. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–9.19. Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013; 347:f5980.20. Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013; 98:F122–6.21. Gopel W, Kribs A, Hartel C, Avenarius S, Teig N, Groneck P, et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 2015; 104:241–6.22. Krajewski P, Chudzik A, Strzalko-Gloskowska B, Gorska M, Kmiecik M, Wieckowska K, et al. Surfactant administration without intubation in preterm infants with respiratory distress syndrome: our experiences. J Matern Fetal Neonatal Med. 2015; 28:1161–4.23. Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed. 2011; 96:F243–8.24. Bhattacharya S, Read B, McGovern E, da Silva O. High-volume surfactant administration using a minimally invasive technique: experience from a Canadian neonatal intensive care unit. Paediatr Child Health. 2019; 24:313–7.25. Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks). Paediatr Anaesth. 2007; 17:364–9.26. Jones P, Dauger S, Peters MJ. Bradycardia during critical care intubation: mechanisms, significance and atropine. Arch Dis Child. 2012; 97:139–44.27. Kribs A, Roll C, Gopel W, Wieg C, Groneck P, Laux R, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015; 169:723–30.28. Mirnia K, Heidarzadeh M, Hoseini MB, Sadeghnia A, Akrami F, Balila M, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial in Alzahra hospital. Iran J Neonatol. 2013; 4:5–9.29. Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017; 102:F17–23.30. Niemarkt HJ, Kuypers E, Jellema R, Ophelders D, Hutten M, Nikiforou M, et al. Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res. 2014; 76:166–70.31. Ricci F, Bresesti I, LaVerde P, Salomone F, Casiraghi C, Mersanne A, et al. Surfactant lung delivery with LISA and InSurE in adult rabbits with respiratory distress. Pediatr Res. 2021; 90:576–83.32. Vento M, Bohlin K, Herting E, Roehr CC, Dargaville PA. Surfactant administration via thin catheter: a practical guide. Neonatology. 2019; 116:211–26.33. Herting E, Hartel C, Gopel W. Less invasive surfactant administration: best practices and unanswered questions. Curr Opin Pediatr. 2020; 32:228–34.34. Bohlin K, Bouhafs RK, Jarstrand C, Curstedt T, Blennow M, Robertson B. Spontaneous breathing or mechanical ventilation alters lung compliance and tissue association of exogenous surfactant in preterm newborn rabbits. Pediatr Res. 2005; 57(5 Pt 1):624–30.35. van der Burg PS, de Jongh FH, Miedema M, Frerichs I, van Kaam AH. Effect of minimally invasive surfactant therapy on lung volume and ventilation in preterm infants. J Pediatr. 2016; 170:67–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prophylactic Administration of Surfactant with Nasal Continuous Positive Airway Pressure in Preterm Infants with Gestational Age Less than 30 Weeks
- Comparison of minimally invasive surfactant therapy with intubation surfactant administration and extubation for treating preterm infants with respiratory distress syndrome: a randomized clinical trial
- Recent Update in the Treatment of Respiratory Distress Syndrome
- Minimally Invasive Surfactant Therapy
- Update of minimally invasive surfactant therapy