Ann Hepatobiliary Pancreat Surg.
2022 Feb;26(1):76-83. 10.14701/ahbps.21-103.
Early laboratory values after liver transplantation are associated with anastomotic biliary strictures
- Affiliations
-
- 1Division of Gastroenterology, Hepatology, and Nutrition, Virginia Commonwealth University Medical Center, Richmond, VA, United States
- 2Division of Gastroenterology and Hepatology, Hunter Holmes McGuire VA Medical Center, Richmond, VA, United States
- 3Department of Radiology, Virginia Commonwealth University Medical Center, Richmond, VA, United States
- 4Division of Gastroenterology and Hepatology, Kansas City VA Medical Center, Kansas City, MO, United States
- 5Department of Transplant Surgery, Virginia Commonwealth University Medical Center, Richmond, VA, United States
- KMID: 2526835
- DOI: http://doi.org/10.14701/ahbps.21-103
Abstract
- Backgrounds/Aims
The aim of this study was to evaluate longitudinal changes of post-liver transplantation (LT) biliary anatomy and to assess the association of increased laboratory values after LT with the development of post-LT anastomotic biliary stricture (ABS).
Methods
Adult deceased donor LT recipients from 2008 and 2019 were evaluated. ABS was defined after blinded review of endoscopic cholangiograms. Controls were patients who underwent LT for hepatocellular carcinoma who did not have any clinical or biochemical concerns for ABS.
Results
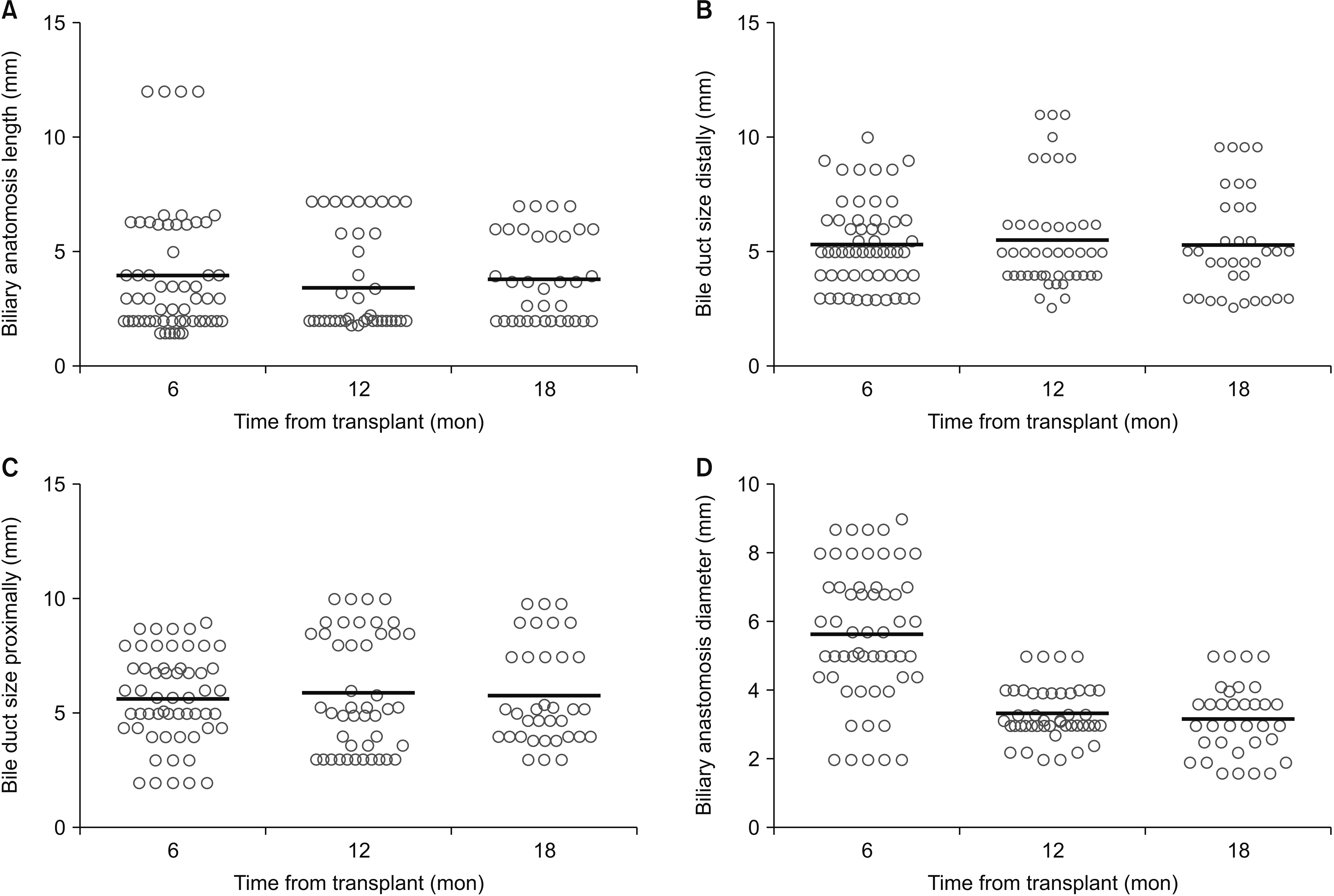
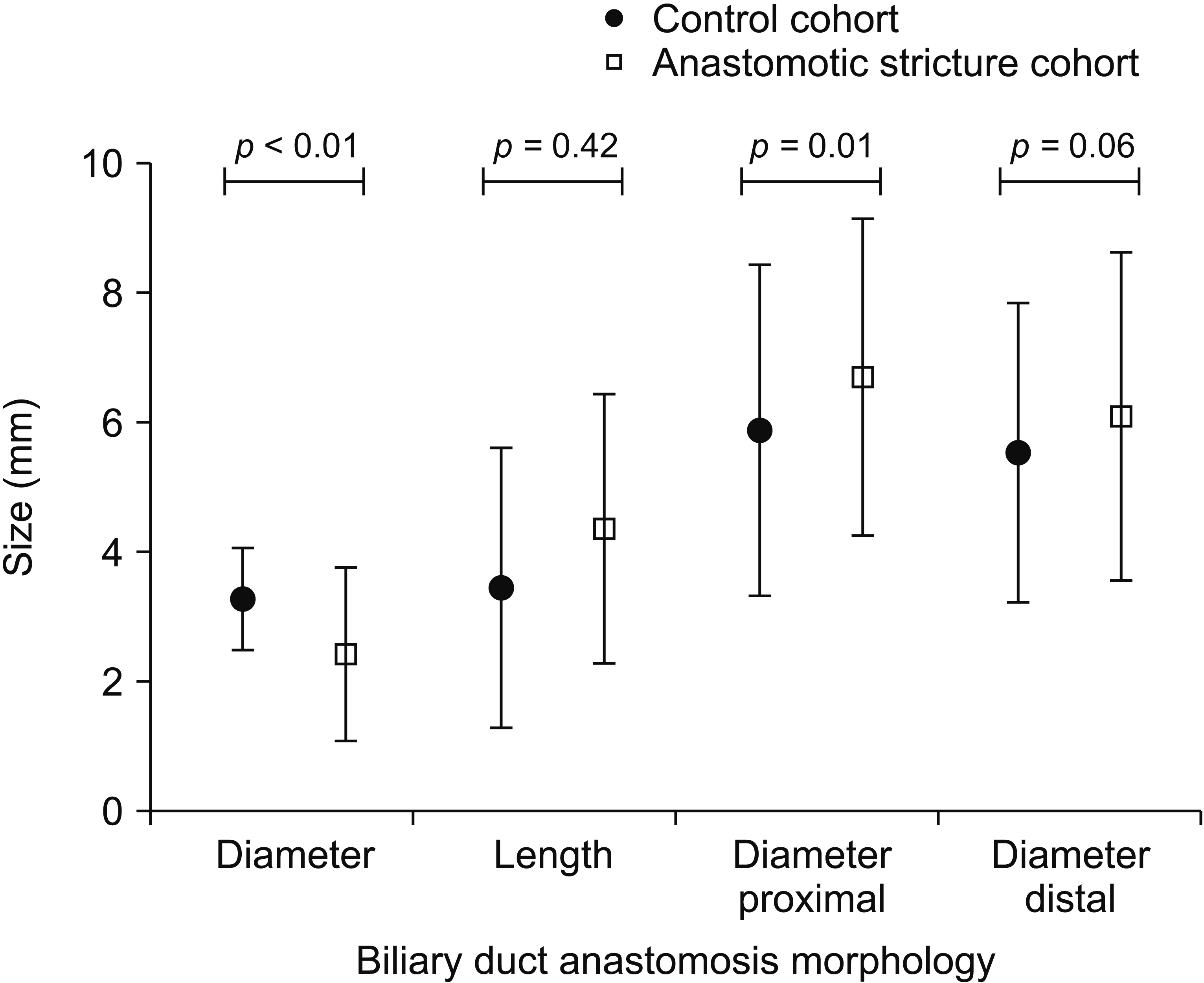
Of 534 patients who underwent LT, 57 patients had ABS and 57 patients served as controls. On MRI, ABS patients had a narrower anastomosis (2.47 ± 1.32 mm vs. 3.38 ± 1.05 mm; p < 0.01) and wider bile duct at 1-cm proximal to the anastomosis (6.73 ± 2.45 mm vs. 5.66 ± 1.95 mm; p = 0.01) than controls. Association between labs at day 7 and ABS formation was as follows: aspartate aminotransferase hazard ratio (HR): 1.014; 95% confidence interval (CI): 1.008–1.020, p = 0.001; total bilirubin HR: 1.292, 95% CI: 1.100–1.517, p = 0.002; and conjugated bilirubin HR: 1.467, 95% CI: 1.216–1.768, p = 0.001. Corresponding analysis results for day 28 were alanine aminotransferase HR: 1.004, 95% CI: 1.002–1.006, p = 0.001; alkaline phosphatase HR: 1.005, 95% CI: 1.003–1.007, p = 0.001; total bilirubin HR: 1.233, 95% CI: 1.110–1.369, p = 0.001; and conjugated bilirubin HR: 1.272, 95% CI: 1.126–1.437, p = 0.001.
Conclusions
Elevation of laboratory values early after LT is associated with ABS formation.
Keyword
Figure
Cited by 1 articles
-
Outcomes of endoscopic retrograde cholangiography and percutaneous transhepatic biliary drainage in liver transplant recipients with a Roux-en-Y biliary-enteric anastomosis
Divyanshoo Rai Kohli, Bashar A. Aqel, Nicole L. Segaran, M. Edwyn Harrison, Norio Fukami, Douglas O. Faigel, Adyr Moss, Amit Mathur, Winston Hewitt, Nitin Katariya, Rahul Pannala
Ann Hepatobiliary Pancreat Surg. 2023;27(1):49-55. doi: 10.14701/ahbps.22-037.
Reference
-
1. Kohli DR, Shah TU, BouHaidar DS, Vachhani R, Siddiqui MS. 2018; Significant infections in liver transplant recipients undergoing endoscopic retrograde cholangiography are few and unaffected by prophylactic antibiotics. Dig Liver Dis. 50:1220–1224. DOI: 10.1016/j.dld.2018.05.014. PMID: 29907534.
Article2. Kohli DR, Harrison ME, Mujahed T, Fukami N, Faigel DO, Pannala R, et al. 2020; Outcomes of endoscopic therapy in donation after cardiac death liver transplant biliary strictures. HPB (Oxford). 22:979–986. DOI: 10.1016/j.hpb.2019.10.018. PMID: 31676256.
Article3. Kohli DR, Desai MV, Kennedy KF, Pandya P, Sharma P. 2021; Patients with post-transplant biliary strictures have significantly higher rates of liver transplant failure and rejection: a nationwide inpatient analysis. J Gastroenterol Hepatol. 36:2008–2014. DOI: 10.1111/jgh.15388. PMID: 33373488.
Article4. Sharma S, Gurakar A, Jabbour N. 2008; Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 14:759–769. DOI: 10.1002/lt.21509. PMID: 18508368.
Article5. Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, et al. 2002; Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 55:224–231. DOI: 10.1067/mge.2002.120813. PMID: 11818927.
Article6. Kohli DR, Vachhani R, Shah TU, BouHaidar DS, Siddiqui MS. 2017; Diagnostic accuracy of laboratory tests and diagnostic imaging in detecting biliary strictures after liver transplantation. Dig Dis Sci. 62:1327–1333. DOI: 10.1007/s10620-017-4515-0. PMID: 28265825.
Article7. Kochhar G, Parungao JM, Hanouneh IA, Parsi MA. 2013; Biliary complications following liver transplantation. World J Gastroenterol. 19:2841–2846. DOI: 10.3748/wjg.v19.i19.2841. PMID: 23704818. PMCID: PMC3660810.
Article8. Kohli DR, Pannala R, Crowell MD, Fukami N, Faigel DO, Aqel BA, et al. 2021; Interobserver agreement for classifying post-liver transplant biliary strictures in donation after circulatory death donors. Dig Dis Sci. 66:231–237. DOI: 10.1007/s10620-020-06169-7. PMID: 32124198.
Article9. Simoes P, Kesar V, Ahmad J. 2015; Spectrum of biliary complications following live donor liver transplantation. World J Hepatol. 7:1856–1865. DOI: 10.4254/wjh.v7.i14.1856. PMID: 26207167. PMCID: PMC4506943.
Article10. Tabibian JH, Girotra M, Yeh HC, Singh VK, Okolo PI III, Cameron AM, et al. 2015; Risk factors for early repeat ERCP in liver transplantation patients with anastomotic biliary stricture. Ann Hepatol. 14:340–347. DOI: 10.1016/S1665-2681(19)31273-6. PMID: 25864214.
Article11. Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, et al. 2013; Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 19:3–26. DOI: 10.1002/lt.23566. PMID: 23281277.
Article12. Nair S, Lingala S, Satapathy SK, Eason JD, Vanatta JM. 2014; Clinical algorithm to guide the need for endoscopic retrograde cholangiopancreatography to evaluate early postliver transplant cholestasis. Exp Clin Transplant. 12:543–547. PMID: 25489806.13. Rao HB, Prakash A, Sudhindran S, Venu RP. 2018; Biliary strictures complicating living donor liver transplantation: problems, novel insights and solutions. World J Gastroenterol. 24:2061–2072. DOI: 10.3748/wjg.v24.i19.2061. PMID: 29785075. PMCID: PMC5960812.
Article14. Woo YS, Lee KH, Lee KT, Lee JK, Kim JM, Kwon CHD, et al. 2017; Postoperative changes of liver enzymes can distinguish between biliary stricture and graft rejection after living donor liver transplantation: a longitudinal study. Medicine (Baltimore). 96:e6892. DOI: 10.1097/MD.0000000000006892. PMID: 28984750. PMCID: PMC5737986.15. Ben-Ari Z, Weiss-Schmilovitz H, Sulkes J, Brown M, Bar-Nathan N, Shaharabani E, et al. 2004; Serum cholestasis markers as predictors of early outcome after liver transplantation. Clin Transplant. 18:130–136. DOI: 10.1046/j.1399-0012.2003.00135.x. PMID: 15016125.
Article16. Bhati C, Idowu MO, Sanyal AJ, Rivera M, Driscoll C, Stravitz RT, et al. 2017; Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation. 101:1867–1874. DOI: 10.1097/TP.0000000000001709. PMID: 28296807.
Article17. Kohli DR, Harrison ME, Adike AO, El Kurdi B, Fukami N, Faigel DO, et al. 2019; Predictors of biliary strictures after liver transplantation among recipients of dcd (donation after cardiac death) grafts. Dig Dis Sci. 64:2024–2030. DOI: 10.1007/s10620-018-5438-0. PMID: 30604376.
Article18. Wroblewski F. 1958; The clinical significance of alterations in transaminase activities of serum and other body fluids. Adv Clin Chem. 1:313–351. DOI: 10.1016/S0065-2423(08)60362-5. PMID: 13571034.19. Hall P, Cash J. 2012; What is the real function of the liver 'function' tests? Ulster Med J. 81:30–36. PMID: 23536736. PMCID: PMC3609680.20. Kamimoto Y, Horiuchi S, Tanase S, Morino Y. 1985; Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 5:367–375. DOI: 10.1002/hep.1840050305. PMID: 3997068.
Article21. Turlin B, Slapak GI, Hayllar KM, Heaton N, Williams R, Portmann B. 1995; Centrilobular necrosis after orthotopic liver transplantation: a longitudinal clinicopathologic study in 71 patients. Liver Transpl Surg. 1:285–289. DOI: 10.1002/lt.500010503. PMID: 9346584.
Article22. Girometti R, Como G, Bazzocchi M, Zuiani C. 2014; Post-operative imaging in liver transplantation: state-of-the-art and future perspectives. World J Gastroenterol. 20:6180–6200. DOI: 10.3748/wjg.v20.i20.6180. PMID: 24876739. PMCID: PMC4033456.
Article23. Thethy S, Thomson BNJ, Pleass H, Wigmore SJ, Madhavan K, Akyol M, et al. 2004; Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 18:647–653. DOI: 10.1111/j.1399-0012.2004.00254.x. PMID: 15516238.
Article24. Le Berre C, Sandborn WJ, Aridhi S, Devignes MD, Fournier L, Smaïl-Tabbone M, et al. 2020; Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 158:76–94.e2. DOI: 10.1053/j.gastro.2019.08.058. PMID: 31593701.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biliary Strictures after Liver Transplantation
- Endoscopic Management of Anastomotic Strictures after Liver Transplantation
- Anastomotic stricture after liver transplantation: It is not Achilles' heel anymore!
- Temporary Placement of Stent Grafts in Postsurgical Benign Biliary Strictures: a Single Center Experience
- Percutaneous intervention for bilioenteric anastomotic strictures: Current strategies and future directions