Anesth Pain Med.
2022 Jan;17(1):1-11. 10.17085/apm.21115.
Remimazolam: pharmacological characteristics and clinical applications in anesthesiology
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2526522
- DOI: http://doi.org/10.17085/apm.21115
Abstract
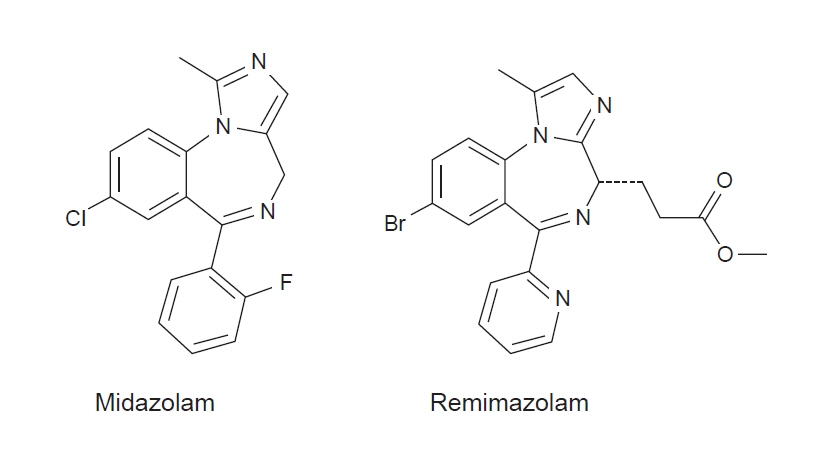
- A novel ultra-short-acting benzodiazepine (BDZ), remimazolam (CNS 7056), has been designed by ‘soft drug’ development to achieve a better sedative profile than that of the current drugs. Notably, the esterase linkage in remimazolam permits rapid hydrolysis to inactivate metabolites by non-specific tissue esterase and induces a unique and favorable pharmacological profile, including rapid onset and offset of sedation and a predictable duration of action. Similar to other BDZs, its sedative effects can be reversed using flumazenil, a BDZ antagonist. The pharmacokinetics and pharmacodynamics of remimazolam are characterized by relatively high clearance, small steady-state volume of distribution, short elimination half-life, short context-sensitive half-life, and fast onset and recovery, indicating rapid elimination, minimal tissue accumulation, and good control. In addition, remimazolam possesses a superior safety profile, including low liability for cardiorespiratory depression and injection pain, making it a preferred hypnotic agent in various clinical settings. Early clinical investigations suggest that remimazolam is well tolerated and effective for procedural sedation and for induction and maintenance of general anesthesia. To date, however, the clinical use of remimazolam has been confined to a few volunteer studies and a limited number of clinical investigations. Therefore, further studies regarding its recovery issues or postoperative complications, characteristics of electroencephalogram changes, and cost-benefit analyses are required to facilitate its widespread use.
Keyword
Figure
Cited by 3 articles
-
Comparison of remimazolam–remifentanil and propofol–remifentanil during laparoscopic cholecystectomy
Tae Young Lee, Min A Kim, Deuk Won Eom, Ji Wook Jung, Chan Jong Chung, Sang Yoong Park
Anesth Pain Med. 2023;18(3):252-259. doi: 10.17085/apm.22252.Comment on "Psychogenic coma after general anesthesia with remimazolam and remifentanil -a case report-"
Saecheol Oh, Dal-ah Kim
Korean J Anesthesiol. 2023;76(5):510-511. doi: 10.4097/kja.23202.A message from the Editor-in-Chief and Editorial Board, 2023: journal metrics and statistics, and appreciation to reviewers
Jun Hyun Kim, Hyun Kang
Anesth Pain Med. 2024;19(1):1-4. doi: 10.17085/apm.24008.
Reference
-
1. Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021; 12:690875.2. Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 1997; 18: 425-9. Erratum in: Trends Pharmacol Sci. 1998; 19:256.3. Goudra BG, Singh PM. Remimazolam: The future of its sedative potential. Saudi J Anaesth. 2014; 8:388–91.4. Egan TD. Is anesthesiology going soft?: trends in fragile pharmacology. Anesthesiology. 2009; 111:229–30.5. Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007; 107:60–6.6. Worthington MT, Antonik LJ, Goldwater DR, Lees JP, Wilhelm-Ogunbiyi K, Borkett KM, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013; 117:1093–100.7. Lee A, Shirley M. Remimazolam: a review in procedural sedation. Drugs. 2021; 81:1193–201.8. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012; 115:274–83.9. Zhou Y, Hu P, Huang Y, Nuoer S, Song K, Wang H, et al. Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative HR7056 in Chinese healthy subjects. Front Pharmacol 2018; 9: 1316. Erratum in: Front Pharmacol. 2019; 10:251.10. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020; 132:636–51.11. Sheng XY, Liang Y, Yang XY, Li LE, Ye X, Zhao X, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020; 76:383–91.12. Sneyd JR. Remimazolam: new beginnings or just a me-too? Anesth Analg. 2012; 115:217–9.13. Freyer N, Knöspel F, Damm G, Greuel S, Schneider C, Seehofer D, et al. Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system. Drug Des Devel Ther. 2019; 13:1033–47.14. Zhou Y, Hu P, Jiang J. Metabolite characterization of a novel sedative drug, remimazolam in human plasma and urine using ultra high-performance liquid chromatography coupled with synapt high-definition mass spectrometry. J Pharm Biomed Anal. 2017; 137:78–83.15. Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. 2020; 34:479–82.16. Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016; 36:1021–7.17. Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012; 115:284–96.18. Doi M. Remimazolam. J Jpn Soc Clin Anesth. 2014; 34:860–6.19. Stöhr T, Colin PJ, Ossig J, Pesic M, Borkett K, Winkle P, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021; 127:415–23.20. Rogers WK, McDowell TS. Remimazolam, a short-acting GABAA receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs. 2010; 13:929–37.21. Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990; 10:244–51.22. Eisenried A, Schüttler J, Lerch M, Ihmsen H, Jeleazcov C. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II. Pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020; 132:652–66.23. Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020; 60:505–14.24. Checketts MR, Alladi R, Ferguson K, Gemmell L, Handy JM, Klein AA, et al. Association of Anaesthetists of Great Britain and Ireland. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2016; 71:85–93.25. Miyake W, Oda Y, Ikeda Y, Hagihira S, Iwaki H, Asada A. Electroencephalographic response following midazolam-induced general anesthesia: relationship to plasma and effect-site midazolam concentrations. J Anesth. 2010; 24:386–93.26. Ibrahim AE, Taraday JK, Kharasch ED. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology. 2001; 95:1151–9.27. Absalom AR, Glen JI, Zwart GJ, Schnider TW, Struys MM. Target-controlled infusion: a mature technology. Anesth Analg. 2016; 122:70–8.28. Sneyd JR. Remifentanil manual versus target-controlled infusion. Anesth Analg. 2003; 97:300; author reply 300.29. Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020; 33:506–11.30. Zhou J, Leonowens C, Ivaturi VD, Lohmer LL, Curd L, Ossig J, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020; 66:109899.31. Hinkelbein J, Lamperti M, Akeson J, Santos J, Costa J, De Robertis E, et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol. 2018; 35:6–24.32. Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020; 12:4594–603.33. Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018; 88:427–37. e6.34. Chen SH, Yuan TM, Zhang J, Bai H, Tian M, Pan CX, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. 2021; 36:474–81.35. Oka S, Satomi H, Sekino R, Taguchi K, Kajiwara M, Oi Y, Kobayashi R. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci. 2021; 63:209–11.36. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol 2021; 21: 156. Erratum in: BMC Anesthesiol. 2021; 21:173.37. Enestvedt BK, Eisen GM, Holub J, Lieberman DA. Is the American Society of Anesthesiologists classification useful in risk stratification for endoscopic procedures? Gastrointest Endosc. 2013; 77:464–71.38. Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis. 2021; 53:94–101.39. PAION, AG. Investigator's brochure remimazolam (CNS 7056). Ver 13.0. Ver 13.0. London: PAION, AG;2018.40. Acacia Pharma. BYFAVOTM (remimazolam): US prescribing information [Internet]. 2021 [2021 Nov 10]. Available from: https://acaciapharma.com/news/2020/07/acacia-pharma-announces-us-fda-approval-of-byfavo-remimazolam-for-injection-for-the-induction-and-maintenance-of-procedural-sedation.41. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020; 34:543–53.42. Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020; 34:491–501.43. Probst S, Bevilacqua C, Eibel S, Müller A, Wahlers S, Soehngen M. Difference in vasopressor use and usage patterns in patients undergoing cardiac surgery with remimazolam versus propofol/sevoflurane for general anesthesia. Paper presented at: Anesthesiology 2015 Annual Meeting; 2015 Oct 24-28; San Diego, CA, USA. Schaumburg (IL): American Society of Anesthesiologists, 2015. p. A4025.44. Hirata N, Hayamizu K, Yamakage M. How to administer remimazolam for anesthesia induction. J Anesth. 2020; 34:962.45. Karavokiros KA, Tsipis GB. Flumazenil: a benzodiazepine antagonist. DICP. 1990; 24:976–81.46. Brogden RN, Goa KL. Flumazenil. A reappraisal of its pharmacological properties and therapeutic efficacy as a benzodiazepine antagonist. Drugs 1991; 42: 1061-89. Erratum in: Drugs. 1992; 43:442.47. Klotz U, Kanto J. Pharmacokinetics and clinical use of flumazenil (Ro 15-1788). Clin Pharmacokinet. 1988; 14:1–12.48. Sivilotti ML. Flumazenil, naloxone and the 'coma cocktail'. Br J Clin Pharmacol. 2016; 81:428–36.49. Yamamoto T, Kurabe M, Kamiya Y. Re-sleeping after reversal of remimazolam by flumazenil. J Anesth. 2021; 35:322.50. Yamamoto T, Kurabe M, Kamiya Y. A mechanism of re-sedation caused by remimazolam. J Anesth. 2021; 35:467–8.51. Kleiman RB, Darpo B, Thorn M, Stoehr T, Schippers F. Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol. 2020; 86:1600–9.52. Chen W, Chen S, Huang Y. Induction and maintenance of procedural sedation in adults: focus on remimazolam injection. Expert Rev Clin Pharmacol. 2021; 14:411–26.53. Schippers F, Pesic M, Saunders R, Borkett K, Searle S, Webster L, et al. Randomized crossover trial to compare abuse liability of intravenous remimazolam versus intravenous midazolam and placebo in recreational central nervous system depressant users. J Clin Pharmacol. 2020; 60:1189–97.54. Sasaki H, Hoshijima H, Mizuta K. Ringer's acetate solution-induced precipitation of remimazolam. Br J Anaesth. 2021; 126:e87–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Patient-controlled sedation using remimazolam during third molar extraction: a case report
- Psychogenic coma after general anesthesia with remimazolam and remifentanil -a case report-
- Remimazolam – current knowledge on a new intravenous benzodiazepine anesthetic agent
- Total intravenous anesthesia with remimazolam in a patient with epilepsy who underwent deep brain stimulation: a case report
- Review of remimazolam and sedatives in the intensive care unit