J Korean Diabetes.
2021 Sep;22(3):207-219. 10.4093/jkd.2021.22.3.207.
Combined Effects of Insulin Resistance and Inflammation on Comorbidities of Type 2 Diabetes
- Affiliations
-
- 1Hyundai Uvis Hospital, Incheon, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 4Huh’s Diabetes Center and the 21st Century Diabetes and Vascular Research Institute, Seoul, Korea
- 5Yonsei Lee Hyun Chul’s Clinic, Seoul, Korea
- KMID: 2526203
- DOI: http://doi.org/10.4093/jkd.2021.22.3.207
Abstract
- Background
Insulin resistance (IR) and inflammation are closely related to each other and share common pathophysiological and metabolic mechanisms. We aimed to investigate the combined effect of IR and inflammation on comorbidities of type 2 diabetes mellitus (T2DM).
Methods
A total 3,758 patients with T2DM were recruited through Huh’s Diabetes Center from January 2003 to June 2009. Insulin sensitivity was measured by a rate constant for plasma glucose disappearance (Kitt , %/min) using short insulin tolerance test. High sensitivity C-reactive protein (hs-CRP) was used as a surrogate for inflammation.
Results
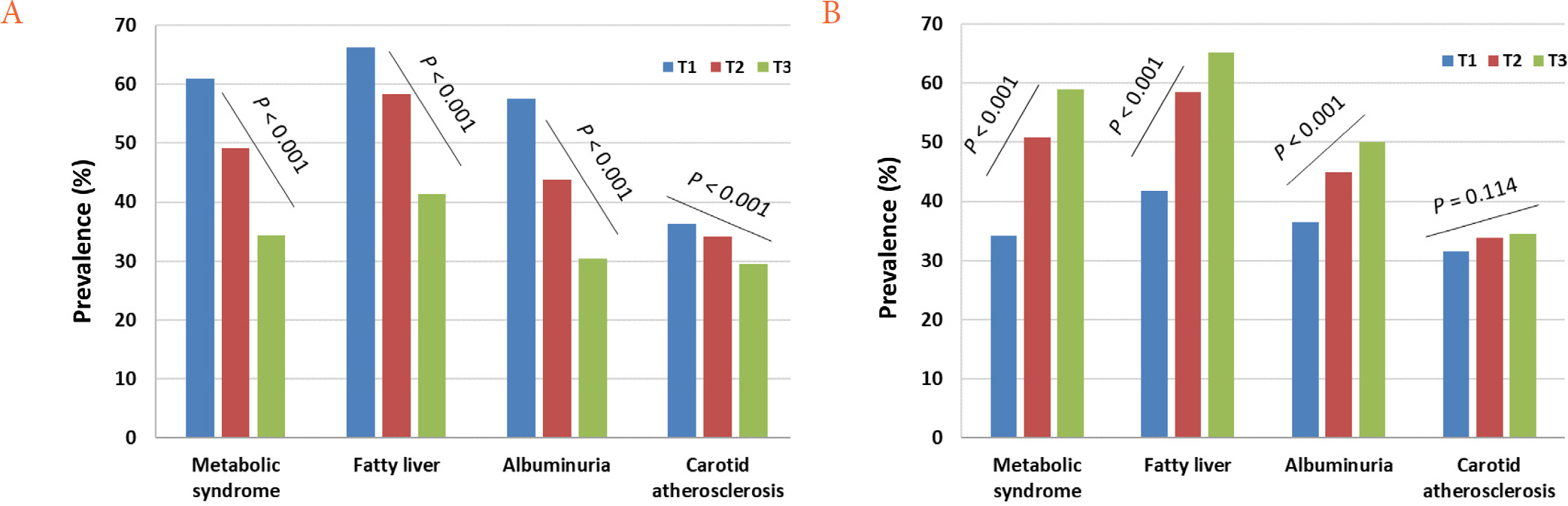
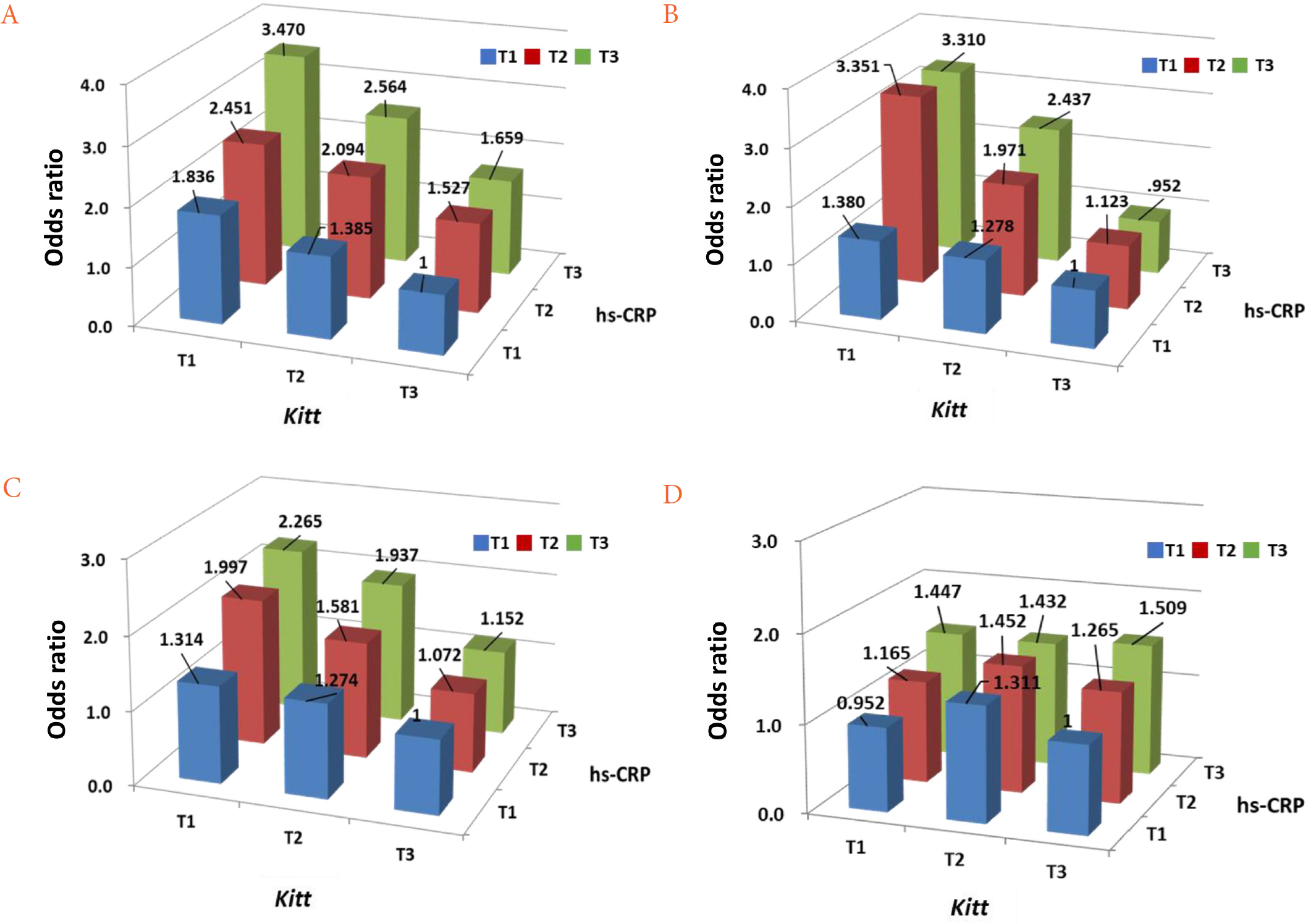
Patients with the lowest tertile of Kitt (IR group) showed worse cardio-metabolic parameters while those with the highest tertile of hs-CRP levels had worse cardio-metabolic parameters. The prevalence of metabolic syndrome, fatty liver, albuminuria, and carotid atherosclerosis decreased with Kitt tertile, but increased with hs-CRP tertile. In multiple regression analysis, both Kitt and hs-CRP were independent risk factors for comorbidities of T2DM. In addition, they showed synergistic effects on these comorbidities.
Conclusion
Both IR and inflammation were significantly associated with comorbidities of T2DM in a dose dependent manner. In addition, the coexistence of IR and inflammation may synergistically contribute to increased comorbidities of T2DM.
Keyword
Figure
Reference
-
1. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011; 11:98–107.
Article2. Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010; 375:132–40.3. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018; 17:122.
Article4. Soumya D, Srilatha B. Late stage complications of diabetes and insulin resistance. J Diabetes Metab. 2011; 2:1000167.
Article5. Sinha SK, Nicholas SB, Sung JH, Correa A, Rajavashisth TB, Norris KC, et al. hs-CRP is associated with incident diabetic nephropathy: findings from the Jackson Heart Study. Diabetes Care. 2019; 42:2083–9.
Article6. Song Y, Yang SK, Kim J, Lee DC. Association between C-reactive protein and metabolic syndrome in Korean adults. Korean J Fam Med. 2019; 40:116–23.
Article7. Lee J, Yoon K, Ryu S, Chang Y, Kim HR. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS One. 2017; 12:e0172666.
Article8. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015; 2015:508409.
Article9. Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018; 128:1538–50.
Article10. Park SW, Kim SK, Cho YW, Kim DJ, Song YD, Choi YJ, et al. Insulin resistance and carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2009; 205:309–13.
Article11. Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Tai TY, et al. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes Care. 2011; 34:982–7.
Article12. Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, et al. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean non-diabetic adults. J Korean Med Sci. 2006; 21:695–700.
Article13. Isokuortti E, Zhou Y, Peltonen M, Bugianesi E, Clement K, Bonnefont-Rousselot D, et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia. 2017; 60:1873–82.
Article14. Hossain IA, Akter S, Bhuiyan FR, Shah MR, Rahman MK, Ali L. Subclinical inflammation in relation to insulin resistance in prediabetic subjects with nonalcoholic fatty liver disease. BMC Res Notes. 2016; 9:266.
Article15. Park SW, Yun YS, Ahn CW, Nam JH, Kwon SH, Song MK, et al. Short insulin tolerance test (SITT) for the determination of in vivo insulin sensitivity-a comparison with euglycemic clamp test. Korean Diabetes J. 1998; 22:199–208.16. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–95.
Article17. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/ National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–52.18. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014; 63:713–35.
Article19. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997; 146:483–94.
Article20. Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000; 108(Suppl 6a):9S–14S.
Article21. Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond). 2010; 7:26.
Article22. Motamed N, Miresmail SJ, Rabiee B, Keyvani H, Farahani B, Maadi M, et al. Optimal cutoff points for HOMA-IR and QUICKI in the diagnosis of metabolic syndrome and non-alcoholic fatty liver disease: a population based study. J Diabetes Complications. 2016; 30:269–74.
Article23. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004; 27:813–23.
Article24. Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne). 2012; 3:170.
Article25. Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013; 62:397–408.26. Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005; 16(Suppl 1):S78–82.
Article27. Kelly DJ, Chanty A, Gow RM, Zhang Y, Gilbert RE. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol. 2005; 16:1654–60.28. Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991; 40:1007–12.
Article29. Kahn SE, Zinman B, Haffner SM, O'Neill MC, Kravitz BG, Yu D, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006; 55:2357–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Resistance and Insulin Resistance Syndrome
- Using Motivational Interviewing to Overcome Psychological Insulin Resistance
- Letter: Effects of Aerobic Exercise Intensity on Insulin Resistance in Patients with Type 2 Diabetes Mellitus (Korean Diabetes J 33(5):401-411, 2009)
- Response: Effects of Aerobic Exercise Intensity on Insulin Resistance in Patients with Type 2 Diabetes Mellitus (Korean Diabetes J 33:(5)401-411, 2009)
- Glut4 in the insulin resistance of NIDDM