J Korean Med Sci.
2022 Feb;37(5):e38. 10.3346/jkms.2022.37.e38.
Nationwide Survey for Current Status of Laboratory Diagnosis of Clostridioides difficile Infection in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Laboratory Medicine, Sanggye Paik Hospital, School of Medicine, Inje University, Seoul, Korea
- 4Infection Control Office, Inje University Sanggye Paik Hospital, Seoul, Korea
- 5Department of Laboratory Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 6Department of Laboratory Medicine, Wonkwang University Hospital, Iksan, Korea
- 7Department of Laboratory Medicine, College of Medicine, Yeungnam University, Daegu, Korea
- 8Department of Laboratory Medicine, Keimyung University School of Medicine, Daegu, Korea
- 9Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 10Department of Laboratory Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- 11Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 12Department of Internal Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea
- 13Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2525490
- DOI: http://doi.org/10.3346/jkms.2022.37.e38
Abstract
- Background
The interest in Clostridioides difficile infection (CDI) has increased, and the choice of assays became wider since the first national survey in Korea on CDI diagnosis in 2015. We conducted a survey of the domestic CDI assays with more varied questions to understand the current situation in Korea.
Methods
In April 2018, about 50 questions on the current status of CDI assays and details on implementation and perceptions were written, and a survey questionnaire was administered to laboratory medicine specialists in 200 institutions.
Results
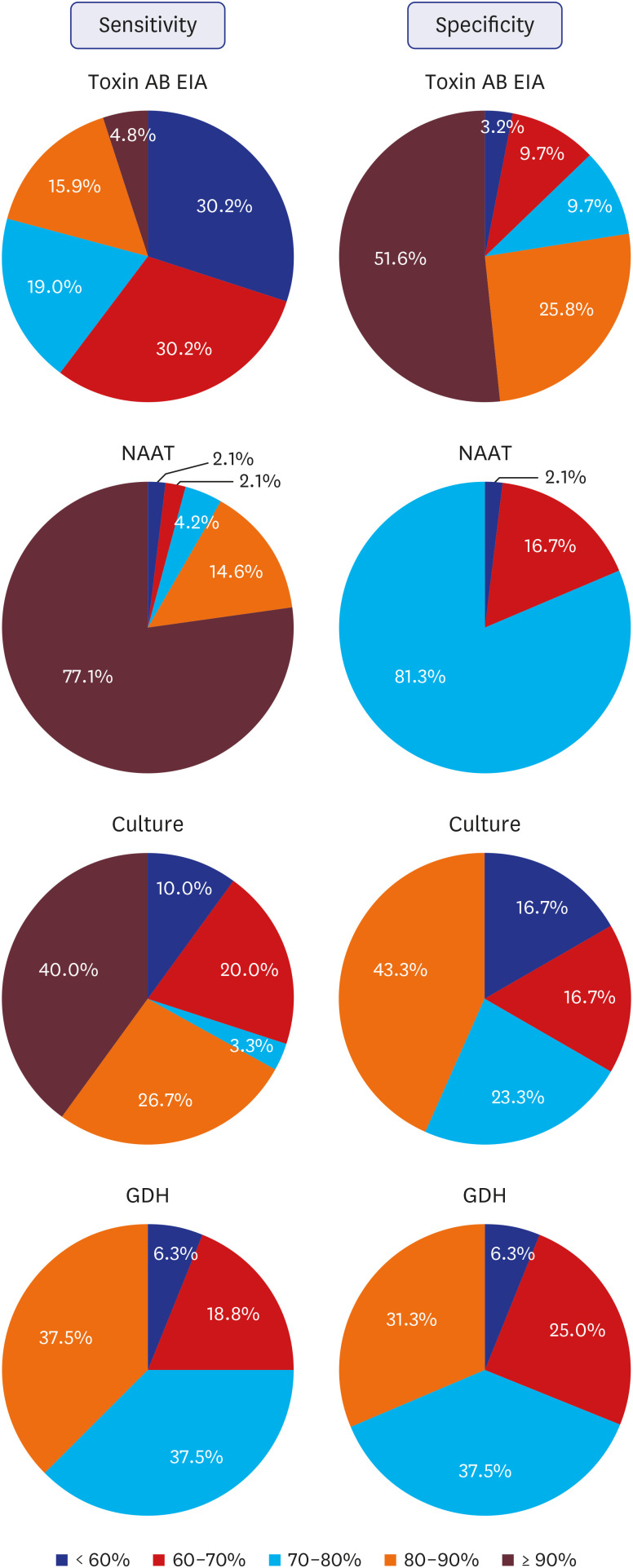
One-hundred and fifty institutions responded to the questionnaire, of which 90 (60.0%) including one commercial laboratory, performed CDI assays. The toxin AB enzyme immunoassay (toxin AB EIA), nucleic acid amplification test (NAAT), and C. difficile culture, glutamate dehydrogenase assay, alone or in combination with other assays, were used in 75 (84.3%), 52 (58.4%), 35 (36.0%), and 23 (25.8%), respectively, and 65 (73.0%) institutions performed a combination of two or more assays. The sensitivity of toxin AB EIA was more negatively perceived, and that on specificity was more positively perceived. The perception of sensitivity and specificity of NAAT was mostly positive. Perception on the algorithm test projected it as useful but in need of countermeasures. Sixty-three (73.3%) institutions responded that they performed surveillance on CDI.
Conclusion
This study provides useful evidence on the current status of CDI laboratory diagnosis in Korea as well as on items that require improvement and is thought to aid in standardizing and improving the CDI laboratory diagnosis in Korea.
Keyword
Figure
Cited by 2 articles
-
Applicability of New Indicators for Healthcare-associated Infections Surveillance in Korea
Sun Hee Park, Sun Young Cho, Soo-Han Choi, Ji Youn Choi, Hee-Jung Son, Hong Bin Kim, Mi Suk Lee
Korean J Healthc Assoc Infect Control Prev. 2022;27(2):104-117. doi: 10.14192/kjicp.2022.27.2.104.A Severe Infection Caused by a White Colony-Producing Strain of
Clostridioides difficile RTC41/ST588
Se Yoon Park, Heejung Kim, Yangsoon Lee
Ann Lab Med. 2024;44(4):375-377. doi: 10.3343/alm.2023.0474.
Reference
-
1. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015; 372(16):1539–1548. PMID: 25875259.2. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015; 313(4):398–408. PMID: 25626036.3. Crobach MJT, Baktash A, Duszenko N, Kuijper EJ. Diagnostic guidance for C. difficile infections. Adv Exp Med Biol. 2018; 1050:27–44. PMID: 29383662.4. Martínez-Meléndez A, Camacho-Ortiz A, Morfin-Otero R, Maldonado-Garza HJ, Villarreal-Treviño L, Garza-González E. Current knowledge on the laboratory diagnosis of Clostridium difficile infection. World J Gastroenterol. 2017; 23(9):1552–1567. PMID: 28321156.5. Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016; 22(Suppl 4):S63–S81. PMID: 27460910.6. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018; 66(7):987–994. PMID: 29562266.7. Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013; 108(4):478–498. PMID: 23439232.8. Schutze GE, Willoughby RE. Committee on Infectious Diseases. American Academy of Pediatrics. Clostridium difficile infection in infants and children. Pediatrics. 2013; 131(1):196–200. PMID: 23277317.9. Chung HS, Park JS, Shin BM. Laboratory diagnosis of Clostridium difficile infection in Korea: the first national survey. Ann Lab Med. 2019; 39(3):317–321. PMID: 30623624.10. Chung HS, Park JS, Shin BM. Laboratory diagnostic methods for Clostridioides difficile infection: the first systematic review and meta-analysis in Korea. Ann Lab Med. 2021; 41(2):171–180. PMID: 33063678.11. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31(5):431–455. PMID: 20307191.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Trend of Clostridioides difficile Infection in Korean Hospitals with the Analysis of Nationwide Sample Cohort
- A Survey of Physicians’ Perceptions of Diagnostic Tests for Clostridioides difficile Infection
- Laboratory Diagnostic Methods for Clostridioides difficile Infection: the First Systematic Review and Meta-analysis in Korea
- Which is the Preferred Regimen for Non-Severe Clostridioides difficile Infection in Korea, Vancomycin or Metronidazole?
- Clostridioides Infection in Patients with Inflammatory Bowel Disease