Diabetes Metab J.
2022 Jan;46(1):140-148. 10.4093/dmj.2021.0023.
Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus
- Affiliations
-
- 1Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- KMID: 2525133
- DOI: http://doi.org/10.4093/dmj.2021.0023
Abstract
- Background
To investigate the association between free fatty acid (FFA) level at mid-pregnancy and large-for-gestational-age (LGA) newborns in women with gestational diabetes mellitus (GDM).
Methods
We enrolled 710 pregnant women diagnosed with GDM from February 2009 to October 2016. GDM was diagnosed by a ‘two-step’ approach with Carpenter and Coustan criteria. We measured plasma lipid profiles including fasting and 2-hour postprandial FFA (2h-FFA) levels at mid-pregnancy. LGA was defined if birthweights of newborns were above the 90th percentile for their gestational age.
Results
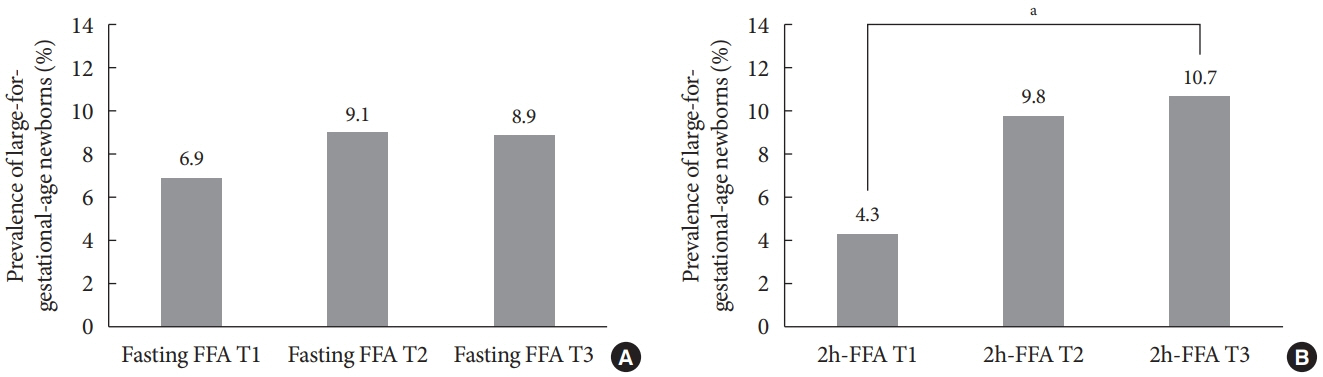
Mean age of pregnant women in this study was 33.1 years. Mean pre-pregnancy body mass index (BMI) was 22.4 kg/m2. The prevalence of LGA was 8.3% (n=59). Levels of 2h-FFA were higher in women who delivered LGA newborns than in those who delivered non-LGA newborns (416.7 μEq/L vs. 352.5 μEq/L, P=0.006). However, fasting FFA was not significantly different between the two groups. The prevalence of delivering LGA newborns was increased with increasing tertile of 2h-FFA (T1, 4.3%; T2, 9.8%; T3, 10.7%; P for trend <0.05). After adjustment for maternal age, pre-pregnancy BMI, and fasting plasma glucose, the highest tertile of 2h-FFA was 2.38 times (95% confidence interval, 1.11 to 5.13) more likely to have LGA newborns than the lowest tertile. However, there was no significant difference between groups according to fasting FFA tertiles.
Conclusion
In women with GDM, a high 2h-FFA level (but not fasting FFA) at mid-pregnancy is associated with an increasing risk of delivering LGA newborns.
Keyword
Figure
Cited by 1 articles
-
Fetal Abdominal Obesity Detected at 24 to 28 Weeks of Gestation Persists until Delivery Despite Management of Gestational Diabetes Mellitus (
Diabetes Metab J 2021;45:547-57)
Wonjin Kim, Soo Kyung Park, Yoo Lee Kim
Diabetes Metab J. 2021;45(6):970-971. doi: 10.4093/dmj.2021.0253.
Reference
-
1. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020; 43(Suppl 1):S14–31.2. Kim MH, Kwak SH, Kim SH, Hong JS, Chung HR, Choi SH, et al. Pregnancy outcomes of women additionally diagnosed as gestational diabetes by the International Association of the Diabetes and Pregnancy Study Groups Criteria. Diabetes Metab J. 2019; 43:766–75.
Article3. Hong S, Lee SM, Kwak SH, Kim BJ, Koo JN, Oh IH, et al. A comparison of predictive performances between old versus new criteria in a risk-based screening strategy for gestational diabetes mellitus. Diabetes Metab J. 2020; 44:726–36.
Article4. Kang S, Kim MH, Kim MY, Hong JS, Kwak SH, Choi SH, et al. Progression to gestational diabetes mellitus in pregnant women with one abnormal value in repeated oral glucose tolerance tests. Diabetes Metab J. 2019; 43:607–14.
Article5. Kim KS, Park SW, Cho YW, Kim SK. Vitamin D deficiency at mid-pregnancy is associated with a higher risk of postpartum glucose intolerance in women with gestational diabetes mellitus. Endocrinol Metab (Seoul). 2020; 35:97–105.
Article6. Oh TJ, Kim YG, Kang S, Moon JH, Kwak SH, Choi SH, et al. Oral glucose tolerance testing allows better prediction of diabetes in women with a history of gestational diabetes mellitus. Diabetes Metab J. 2019; 43:342–9.
Article7. Falcone V, Kotzaeridi G, Breil MH, Rosicky I, Stopp T, Yerlikaya-Schatten G, et al. Early assessment of the risk for gestational diabetes mellitus: can fasting parameters of glucose metabolism contribute to risk prediction? Diabetes Metab J. 2019; 43:785–93.
Article8. Morettini M, Castriota C, Gobl C, Kautzky-Willer A, Pacini G, Burattini L, et al. Glucose effectiveness from short insulinmodified IVGTT and its application to the study of women with previous gestational diabetes mellitus. Diabetes Metab J. 2020; 44:286–94.
Article9. Di Cianni G, Lacaria E, Lencioni C, Resi V. Preventing type 2 diabetes and cardiovascular disease in women with gestational diabetes: the evidence and potential strategies. Diabetes Res Clin Pract. 2018; 145:184–92.10. Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019; 42:372–80.11. Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. 2010; 27:436–41.
Article12. Herrera E, Munilla MA. Maternal lipid metabolism and its implications for fetal growth. Placental function and fetal nutrition Nestle Nutrition Workshop Series. Available from: https://www.nestlenutrition-institute.org/sites/default/files/documents-library/publications/secured/8a57f88112fde6fe164baf24e1454918.pdf (cited 2021 Jul 8).13. Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2010; 89:700–4.
Article14. Harper LM, Mele L, Landon MB, Carpenter MW, Ramin SM, Reddy UM, et al. Carpenter-Coustan compared with national diabetes data group criteria for diagnosing gestational diabetes. Obstet Gynecol. 2016; 127:893–8.
Article15. Hong J. A study on mortality level of newborns and birthweight by gestational age: based on intrauterine growth percentile curve in Korean 1999 birth cohort [master’s thesis]. Seoul: Yonsei University;2003.16. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–95.
Article17. Wikstrom I, Axelsson O, Bergstrom R. Maternal factors associated with high birth weight. Acta Obstet Gynecol Scand. 1991; 70:55–61.
Article18. Lazer S, Biale Y, Mazor M, Lewenthal H, Insler V. Complications associated with the macrosomic fetus. J Reprod Med. 1986; 31:501–5.19. Wikstrom I, Axelsson O, Bergstrom R, Meirik O. Traumatic injury in large-for-date infants. Acta Obstet Gynecol Scand. 1988; 67:259–64.
Article20. Spellacy WN, Miller S, Winegar A, Peterson PQ. Macrosomia: maternal characteristics and infant complications. Obstet Gynecol. 1985; 66:158–61.21. Oral E, Cagdas A, Gezer A, Kaleli S, Aydinli K, Ocer F. Perinatal and maternal outcomes of fetal macrosomia. Eur J Obstet Gynecol Reprod Biol. 2001; 99:167–71.
Article22. Meshari AA, De Silva S, Rahman I. Fetal macrosomia: maternal risks and fetal outcome. Int J Gynaecol Obstet. 1990; 32:215–22.23. Dietz WH. Overweight in childhood and adolescence. N Engl J Med. 2004; 350:855–7.
Article24. Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004; 104:720–6.
Article25. Kramer MS, Morin I, Yang H, Platt RW, Usher R, McNamara H, et al. Why are babies getting bigger?: temporal trends in fetal growth and its determinants. J Pediatr. 2002; 141:538–42.
Article26. Choi SK, Park IY, Shin JC. The effects of pre-pregnancy body mass index and gestational weight gain on perinatal outcomes in Korean women: a retrospective cohort study. Reprod Biol Endocrinol. 2011; 9:6.
Article27. Freinkel N. Banting lecture 1980. Of pregnancy and progeny. Diabetes. 1980; 29:1023–35.
Article28. Kulkarni SR, Kumaran K, Rao SR, Chougule SD, Deokar TM, Bhalerao AJ, et al. Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care. 2013; 36:2706–13.29. Adank MC, Benschop L, Kors AW, Peterbroers KR, Smak Gregoor AM, Mulder MT, et al. Maternal lipid profile in early pregnancy is associated with foetal growth and the risk of a child born large-for-gestational age: a population-based prospective cohort study: maternal lipid profile in early pregnancy and foetal growth. BMC Med. 2020; 18:276.30. Schaefer-Graf UM, Pawliczak J, Passow D, Hartmann R, Rossi R, Buhrer C, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005; 28:1745–50.
Article31. Wang D, Xu S, Chen H, Zhong L, Wang Z. The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol (Oxf). 2015; 83:490–7.
Article32. Barat S, Ghanbarpour A, Bouzari Z, Batebi Z. Triglyceride to HDL cholesterol ratio and risk for gestational diabetes and birth of a large-for-gestational-age newborn. Caspian J Intern Med. 2018; 9:368–75.33. Sieber J, Jehle AW. Free fatty acids and their metabolism affect function and survival of podocytes. Front Endocrinol (Lausanne). 2014; 5:186.
Article34. Roden M, Krssak M, Stingl H, Gruber S, Hofer A, Furnsinn C, et al. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes. 1999; 48:358–64.
Article35. Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003; 284:E863–73.36. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989; 83:1168–73.
Article37. Boden G. Obesity, insulin resistance, type 2 diabetes and free fatty acids. Expert Rev Endocrinol Metab. 2006; 1:499–505.
Article38. Boden G. Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians. 1999; 111:241–8.
Article39. Hawkins M, Tonelli J, Kishore P, Stein D, Ragucci E, Gitig A, et al. Contribution of elevated free fatty acid levels to the lack of glucose effectiveness in type 2 diabetes. Diabetes. 2003; 52:2748–58.
Article40. Sivan E, Boden G. Free fatty acids, insulin resistance, and pregnancy. Curr Diab Rep. 2003; 3:319–22.
Article41. Frayn KN, Summers LK, Fielding BA. Regulation of the plasma non-esterified fatty acid concentration in the postprandial state. Proc Nutr Soc. 1997; 56:713–21.
Article42. Frayn KN, Williams CM, Arner P. Are increased plasma nonesterified fatty acid concentrations a risk marker for coronary heart disease and other chronic diseases? Clin Sci (Lond). 1996; 90:243–53.
Article43. Wang Y, Meng X, Deng X, Okekunle AP, Wang P, Zhang Q, et al. Postprandial saturated fatty acids increase the risk of type 2 diabetes: a cohort study in a Chinese population. J Clin Endocrinol Metab. 2018; 103:1438–46.
Article44. Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007; 61:1145–61.
Article45. Chu X, Liu L, Na L, Lu H, Li S, Li Y, et al. Sterol regulatory element-binding protein-1c mediates increase of postprandial stearic acid, a potential target for improving insulin resistance, in hyperlipidemia. Diabetes. 2013; 62:561–71.
Article46. Liu L, Chu X, Na L, Yuan F, Li Y, Sun C. Decreasing high postprandial stearic acid in impaired fasting glucose by dietary regulation. Eur J Clin Nutr. 2016; 70:795–801.
Article