Diabetes Metab J.
2022 Jan;46(1):71-80. 10.4093/dmj.2020.0274.
Efficacy and Safety of Self-Titration Algorithms of Insulin Glargine 300 units/mL in Individuals with Uncontrolled Type 2 Diabetes Mellitus (The Korean TITRATION Study): A Randomized Controlled Trial
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 4Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
- 5Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2525127
- DOI: http://doi.org/10.4093/dmj.2020.0274
Abstract
- Background
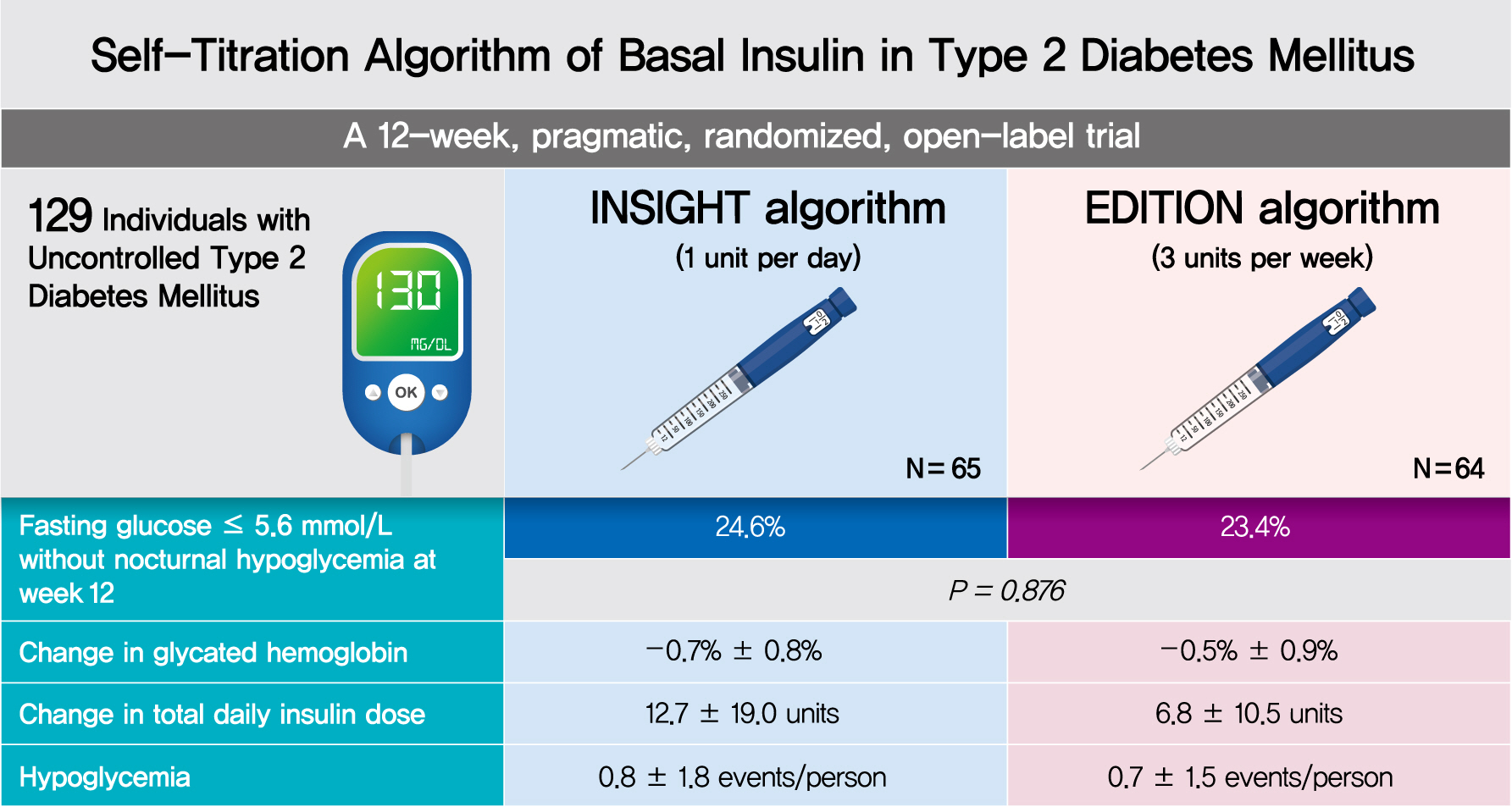
To compare the efficacy and safety of two insulin self-titration algorithms, Implementing New Strategies with Insulin Glargine for Hyperglycemia Treatment (INSIGHT) and EDITION, for insulin glargine 300 units/mL (Gla-300) in Korean individuals with uncontrolled type 2 diabetes mellitus (T2DM).
Methods
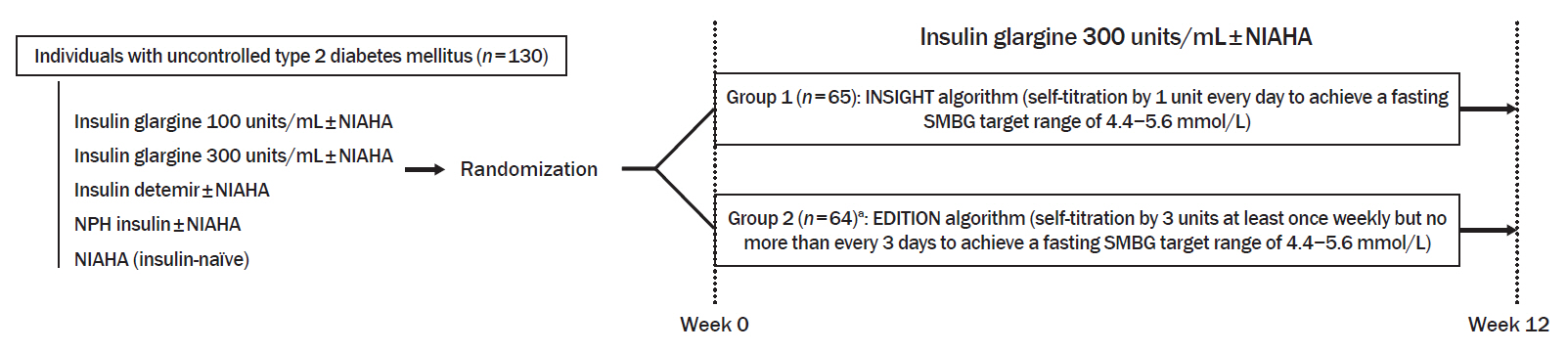
In a 12-week, randomized, open-label trial, individuals with uncontrolled T2DM requiring basal insulin were randomized to either the INSIGHT (adjusted by 1 unit/day) or EDITION (adjusted by 3 units/week) algorithm to achieve a fasting self-monitoring of blood glucose (SMBG) in the range of 4.4 to 5.6 mmol/L. The primary outcome was the proportion of individuals achieving a fasting SMBG ≤5.6 mmol/L without noct urnal hypoglycemia at week 12.
Results
Of 129 individuals (age, 64.1±9.5 years; 66 [51.2%] women), 65 and 64 were randomized to the INSIGHT and EDITION algorithms, respectively. The primary outcome of achievement was comparable between the two groups (24.6% vs. 23.4%, P=0.876). Compared with the EDITION group, the INSIGHT group had a greater reduction in 7-point SMBG but a similar decrease in fasting plasma glucose and glycosylated hemoglobin. The increment of total daily insulin dose was significantly higher in the INSIGHT group than in the EDITION group (between-group difference: 5.8±2.7 units/day, P=0.033). However, body weight was significantly increased only in the EDITION group (0.6±2.4 kg, P=0.038). There was no difference in the occurrence of hypoglycemia between the two groups. Patient satisfaction was significantly increased in the INSIGHT group (P=0.014).
Conclusion
The self-titration of Gla-300 using the INSIGHT algorithm was effective and safe compared with that using the EDITION algorithm in Korean individuals with uncontrolled T2DM (ClinicalTrials.gov number: NCT03406663).
Keyword
Figure
Reference
-
1. U.S. Food and Drug Administration: Prescribing information for TOUJEO 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206538s012lbl.pdf (cited 2021 May 25).2. Shiramoto M, Eto T, Irie S, Fukuzaki A, Teichert L, Tillner J, et al. Single-dose new insulin glargine 300U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015; 17:254–60.
Article3. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units · mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units · mL-1. Diabetes Care. 2015; 38:637–43.4. Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014; 37:2755–62.
Article5. Yki-Jarvinen H, Bergenstal R, Ziemen M, Wardecki M, Muehlen-Bartmer I, Boelle E, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014; 37:3235–43.
Article6. Bolli GB, Riddle MC, Bergenstal RM, Ziemen M, Sestakauskas K, Goyeau H, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015; 17:386–94.
Article7. Terauchi Y, Koyama M, Cheng X, Takahashi Y, Riddle MC, Bolli GB, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2). Diabetes Obes Metab. 2016; 18:366–74.
Article8. Home PD, Bergenstal RM, Bolli GB, Ziemen M, Rojeski M, Espinasse M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015; 38:2217–25.
Article9. Matsuhisa M, Koyama M, Cheng X, Takahashi Y, Riddle MC, Bolli GB, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016; 18:375–83.
Article10. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013; 1281:64–91.
Article11. Yagihashi S, Inaba W, Mizukami H. Dynamic pathology of islet endocrine cells in type 2 diabetes: β-Cell growth, death, regeneration and their clinical implications. J Diabetes Investig. 2016; 7:155–65.
Article12. Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care. 2000; 23:1353–8.
Article13. Chiu KC, Chuang LM, Yoon C. Comparison of measured and estimated indices of insulin sensitivity and beta cell function: impact of ethnicity on insulin sensitivity and beta cell function in glucose-tolerant and normotensive subjects. J Clin Endocrinol Metab. 2001; 86:1620–5.14. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015; 6:495–507.15. Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas: the Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006; 23:736–42.
Article16. Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2020 executive summary. Endocr Pract. 2020; 26:107–39.17. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020; 43(Suppl 1):S98–110.18. International Diabetes Federation Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels: IDF;2012.19. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43:398–406.
Article20. Yale JF, Berard L, Groleau M, Javadi P, Stewart J, Harris SB. TITRATION: a randomized study to assess 2 treatment algorithms with new insulin glargine 300 units/mL. Can J Diabetes. 2017; 41:478–84.
Article21. Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009; 94:709–28.
Article22. Bradley C. Handbook of psychology and diabetes. Chur: Harwood;1994. Chapter 7, Diabetes treatment satisfaction questionnaire. p. 111–32.23. Bradley C. Diabetes treatment satisfaction questionnaire: change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care. 1999; 22:530–2.
Article24. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015; 350:h2147.
Article25. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010; 340:c332.
Article26. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014; 383:1068–83.
Article27. Fonseca VA, Haggar MA. Achieving glycaemic targets with basal insulin in T2DM by individualizing treatment. Nat Rev Endocrinol. 2014; 10:276–81.
Article28. Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018; 20:488–96.
Article29. Russell-Jones D, Dauchy A, Delgado E, Dimitriadis G, Frandsen HA, Popescu L, et al. Take control: a randomized trial evaluating the efficacy and safety of self-versus physician-managed titration of insulin glargine 300U/mL in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019; 21:1615–24.30. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005; 28:1282–8.31. Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003; 26:3080–6.32. Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008; 51:408–16.
Article33. Khunti K, Damci T, Meneghini L, Pan CY, Yale JF; SOLVE Study Group. Study of Once Daily Levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012; 14:654–61.
Article34. Khunti K, Caputo S, Damci T, Dzida GJ, Ji Q, Kaiser M, et al. The safety and efficacy of adding once-daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012; 14:1129–36.
Article35. Meneghini LF, Mauricio D, Orsi E, Lalic NM, Cali AMG, Westerbacka J, et al. The Diabetes Unmet Need with Basal Insulin Evaluation (DUNE) study in type 2 diabetes: achieving HbA1c targets with basal insulin in a real-world setting. Diabetes Obes Metab. 2019; 21:1429–36.
Article36. Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005; 28:2673–9.
Article37. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012; 29:682–9.
Article38. Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017; 11:3–12.
Article39. Brown RJ, Wijewickrama RC, Harlan DM, Rother KI. Uncoupling intensive insulin therapy from weight gain and hypoglycemia in type 1 diabetes. Diabetes Technol Ther. 2011; 13:457–60.
Article40. Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: a systematic review. Diabet Med. 2013; 30:512–24.
Article41. Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006; 166:1836–41.
Article42. Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins-Jones S, Rubin RR, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012; 35:1279–84.
Article43. Patel S, Abreu M, Tumyan A, Adams-Huet B, Li X, Lingvay I. Effect of medication adherence on clinical outcomes in type 2 diabetes: analysis of the SIMPLE study. BMJ Open Diabetes Res Care. 2019; 7:e000761.
Article44. Miya A, Nakamura A, Miyoshi H, Cho KY, Nagai S, Kurihara Y, et al. Satisfaction of switching to combination therapy with lixisenatide and basal insulin in patients with type 2 diabetes receiving multiple daily insulin injection therapy: a randomized controlled trial. J Diabetes Investig. 2018; 9:119–26.
Article45. Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016; 39:2126–40.
Article46. Polonsky WH, Hajos TR, Dain MP, Snoek FJ. Are patients with type 2 diabetes reluctant to start insulin therapy?: an examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011; 27:1169–74.
Article47. Boels AM, Vos RC, Hermans TG, Zuithoff NP, Muller N, Khunti K, et al. What determines treatment satisfaction of patients with type 2 diabetes on insulin therapy?: an observational study in eight European countries. BMJ Open. 2017; 7:e016180.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Efficacy and Safety of Insulin Detemir Administered Once Daily According to Two Titration Algorithms (3-0-3 and 2-4-6-8) in Patients with Type 2 Diabetes Mellitus
- Practical Focus on American Diabetes Association/European Association for the Study of Diabetes Consensus Algorithm in Patients with Type 2 Diabetes Mellitus: Timely Insulin Initiation and Titration (Iran-AFECT)
- Effect of Insulin Glargine in Adolescents with Uncontrolled type 1 Diabetes Mellitus
- Therapeutic Efficacy of Combined Therapy with Once Daily Insulin Glargine and Once Daily Glimepiride in Korean Type 2 Diabetic Patients
- Exenatide versus Insulin Lispro Added to Basal Insulin in a Subgroup of Korean Patients with Type 2 Diabetes Mellitus