Intest Res.
2022 Jan;20(1):150-155. 10.5217/ir.2020.00148.
Granulocyte and monocyte apheresis therapy for patients with active ulcerative colitis associated with COVID-19: a case report
- Affiliations
-
- 1Institute of Gastroenterology, Tokyo Women’s Medical University, Tokyo, Japan
- 2Department of Respiratory Medicine, Tokyo Women’s Medical University, Tokyo, Japan
- 3Division of Nephrology, Department of Medicine, Tokyo Women’s Medical University, Tokyo, Japan
- 4Department of Blood Purification, Tokyo Women’s Medical University, Tokyo, Japan
- 5Primary Care Center, Tokyo Women’s Medical University, Tokyo, Japan
- KMID: 2525082
- DOI: http://doi.org/10.5217/ir.2020.00148
Abstract
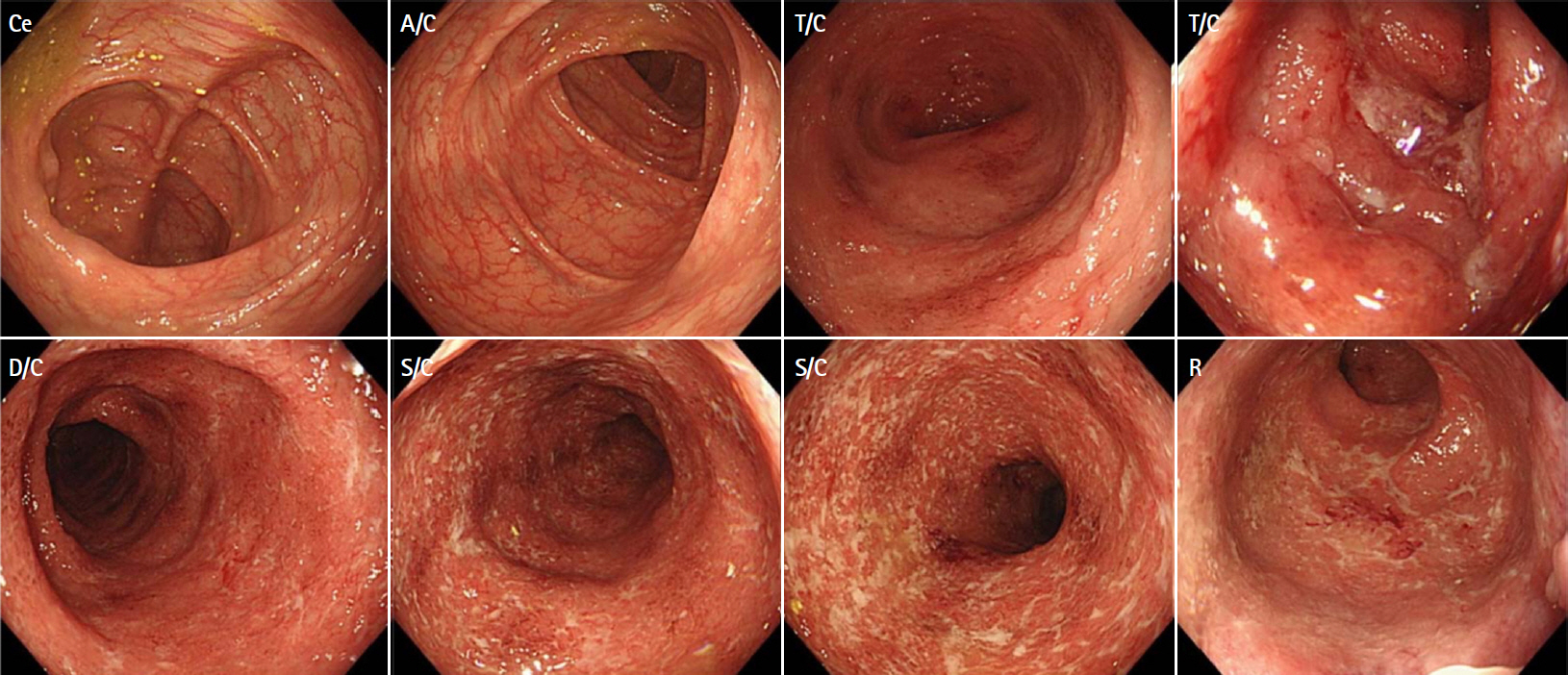
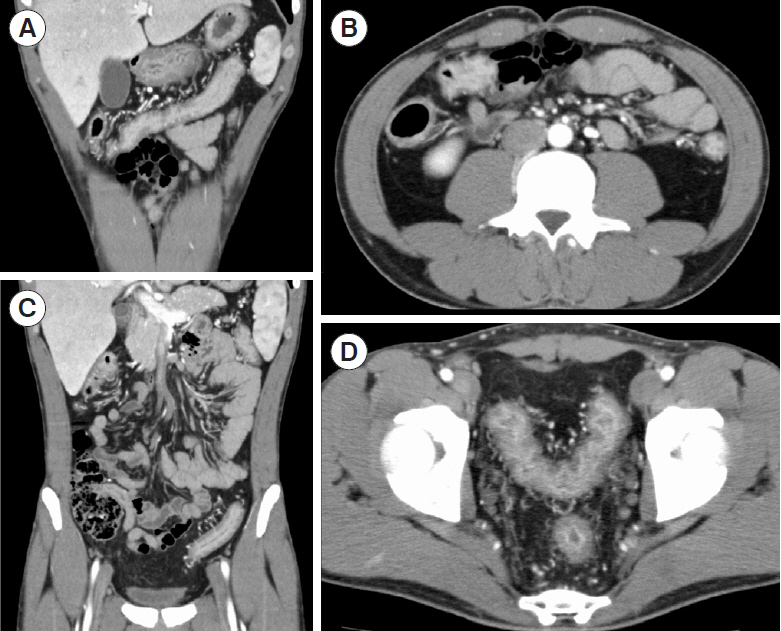
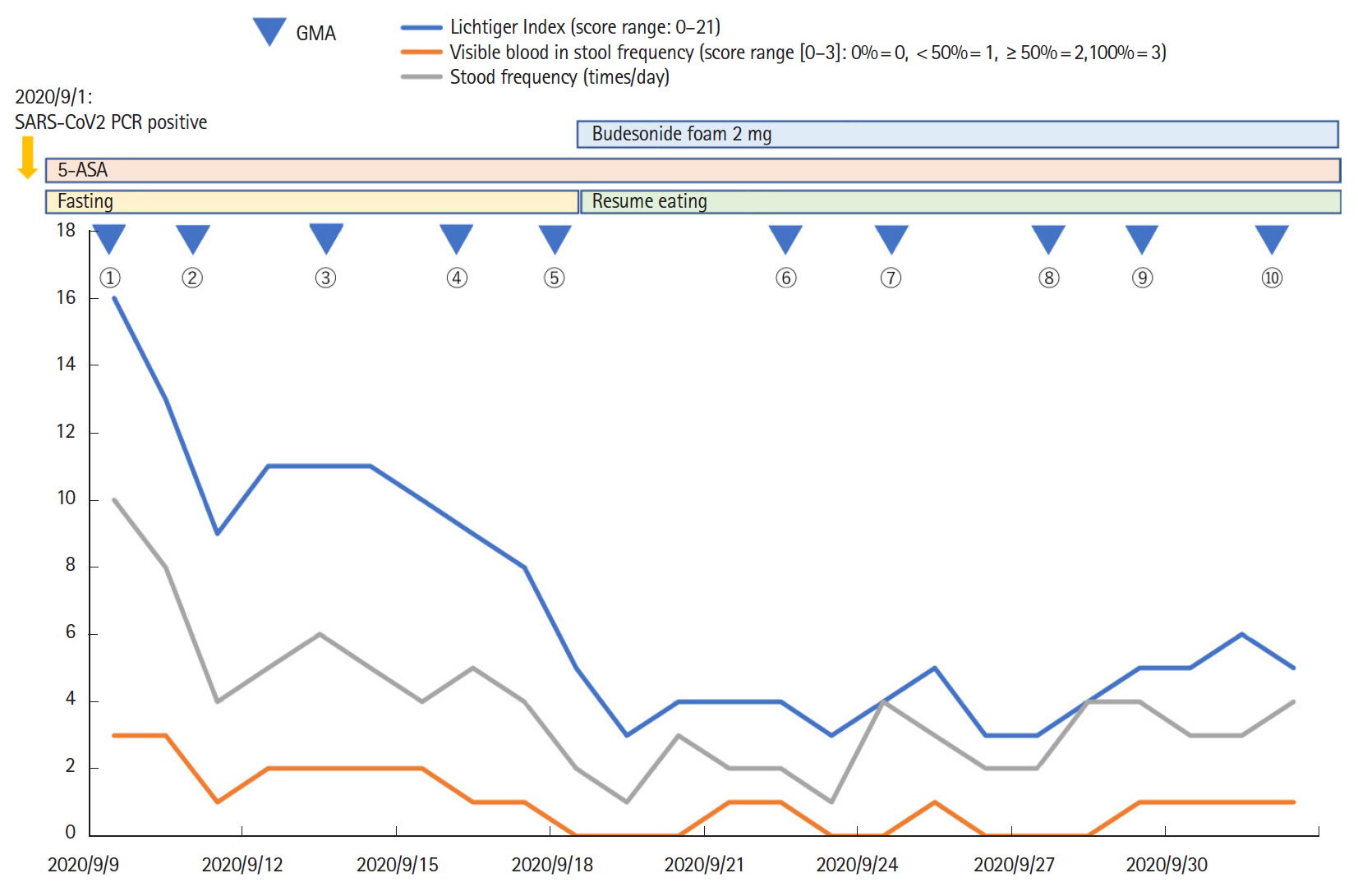
- Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is now a pandemic. Although several treatment guidelines have been proposed for patients who have both inflammatory bowel disease and COVID-19, immunosuppressive therapy is essentially not recommended, and the treatment options are limited. Even in the COVID-19 pandemic, adjuvant adsorptive granulocyte and monocyte apheresis may safely bring ulcerative colitis (UC) into remission by removing activated myeloid cells without the use of immunosuppressive therapy. Our patient was a 25-year-old Japanese male with UC and COVID-19. This is the first case report of the induction of UC remission with granulocyte and monocyte apheresis treatment for active UC associated with COVID-19.
Figure
Cited by 2 articles
-
SARS-CoV-2 Vaccination for Adult Patients with Inflammatory Bowel Disease: Expert Consensus Statements by KASID
Yoo Jin Lee, Seong-Eun Kim, Yong Eun Park, Ji Young Chang, Hyun Joo Song, Duk Hwan Kim, Young Joo Yang, Byung Chang Kim, Jae Gon Lee, Hee Chan Yang, Miyoung Choi, Seung-Jae Myung
Korean J Gastroenterol. 2021;78(2):117-128. doi: 10.4166/kjg.2021.110.SARS-CoV-2 vaccination for adult patients with inflammatory bowel disease: expert consensus statement by KASID
Yoo Jin Lee, Seong-Eun Kim, Yong Eun Park, Ji Young Chang, Hyun Joo Song, Duk Hwan Kim, Young Joo Yang, Byung Chang Kim, Jae Gon Lee, Hee Chan Yang, Miyoung Choi, Seung-Jae Myung
Intest Res. 2022;20(2):171-183. doi: 10.5217/ir.2021.00098.
Reference
-
1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579:270–273.2. Nakase H, Matsumoto T, Matsuura M, et al. Expert opinions on the current therapeutic management of inflammatory bowel disease during the COVID-19 pandemic: Japan IBD COVID-19 Taskforce, Intractable Diseases, the Health and Labor Sciences Research. Digestion. 2021; 102:814–822.
Article3. Magro F, Rahier JF, Abreu C, et al. Inflammatory bowel disease management during the COVID-19 outbreak: the ten do’s and don’ts from the ECCO-COVID taskforce. J Crohns Colitis. 2020; 14:S798–S806.
Article4. Saniabadi AR, Hanai H, Takeuchi K, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003; 7:48–59.
Article5. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article6. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article7. Kappelman MD, Brenner EJ, Colombel JF, et al. SECURE-IBD Database Public Data Update [Internet]. c2020 [cited 2020 Nov 7]. https://covidibd.org/current-data.8. Kashiwagi N, Sugimura K, Koiwai H, et al. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002; 47:1334–1341.9. Sands BE, Sandborn WJ, Feagan B, et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008; 135:400–409.
Article10. Zhu M, Xu X, Nie F, Tong J, Xiao S, Ran Z. The efficacy and safety of selective leukocytapheresis in the treatment of ulcerative colitis: a meta-analysis. Int J Colorectal Dis. 2011; 26:999–1007.
Article11. Yamamoto T, Iida T, Ikeya K, et al. A multicenter retrospective study aiming to identify patients who respond well to adsorptive granulomonocytapheresis in moderately to severely active ulcerative colitis. Clin Transl Gastroenterol. 2018; 9:170.
Article12. Sakuraba A, Motoya S, Watanabe K, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009; 104:2990–2995.
Article13. Yoshino T, Nakase H, Minami N, et al. Efficacy and safety of granulocyte and monocyte adsorption apheresis for ulcerative colitis: a meta-analysis. Dig Liver Dis. 2014; 46:219–226.
Article14. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994; 330:1841–1845.
Article15. Ng SC, Mak JWY, Hitz L, Chowers Y, Bernstein CN, Silverberg MS. COVID-19 pandemic: which IBD patients need to be scoped-who gets scoped now, who can wait, and how to resume to normal. J Crohns Colitis. 2020; 14:S791–S797.
Article16. Shinzaki S, Matsuoka K, Iijima H, et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis. 2017; 11:84–91.
Article17. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020; 8:e46–e47.
Article18. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506.
Article19. Okeke F, Mone A, Swaminath A. The course of SARS-COV2 infection was not severe in a Crohn’s patient who administered maintenance anti-TNF therapy overlapping the early pre-symptomatic period of infection. Antibodies (Basel). 2020; 9:42.
Article20. Kanekura T, Kawahara K. Adsorptive granulocyte and monocyte apheresis: a potentially relevant therapeutic option for COVID-19. Int J Infect Dis. 2020; 99:1–2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Adsorptive Granulocyte and Monocyte Apheresis Effective as an Alternative Treatment Option in Patients with Ulcerative Colitis?
- Adsorptive Granulocyte/Monocyte Apheresis for the Maintenance of Remission in Patients with Ulcerative Colitis: A Prospective Randomized, Double Blind, Sham-Controlled Clinical Trial
- Adsorptive Granulocyte and Monocyte Apheresis in the Treatment of Ulcerative Colitis: The First Multicenter Study in China
- Granulocyte and Monocyte Adsorption Apheresis in Korean Conventional Treatment-refractory Patients with Active Ulcerative Colitis: A Prospective Open-label Multicenter Study
- A Case of Cytomegalvirus Colitis Developed during the Treatment of Ulcerative Colitis