Clin Endosc.
2022 Jan;55(1):77-85. 10.5946/ce.2021.002.
Clinical Outcomes and Adverse Events of Gastric Endoscopic Submucosal Dissection of the Mid to Upper Stomach under General Anesthesia and Monitored Anesthetic Care
- Affiliations
-
- 1Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Statistics and Data Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, South Korea
- KMID: 2525053
- DOI: http://doi.org/10.5946/ce.2021.002
Abstract
- Background/Aims
Endoscopic submucosal dissection (ESD) of gastric tumors in the mid-to-upper stomach is a technically challenging procedure. This study compared the therapeutic outcomes and adverse events of ESD of tumors in the mid-to-upper stomach performed under general anesthesia (GA) or monitored anesthesia care (MAC).
Methods
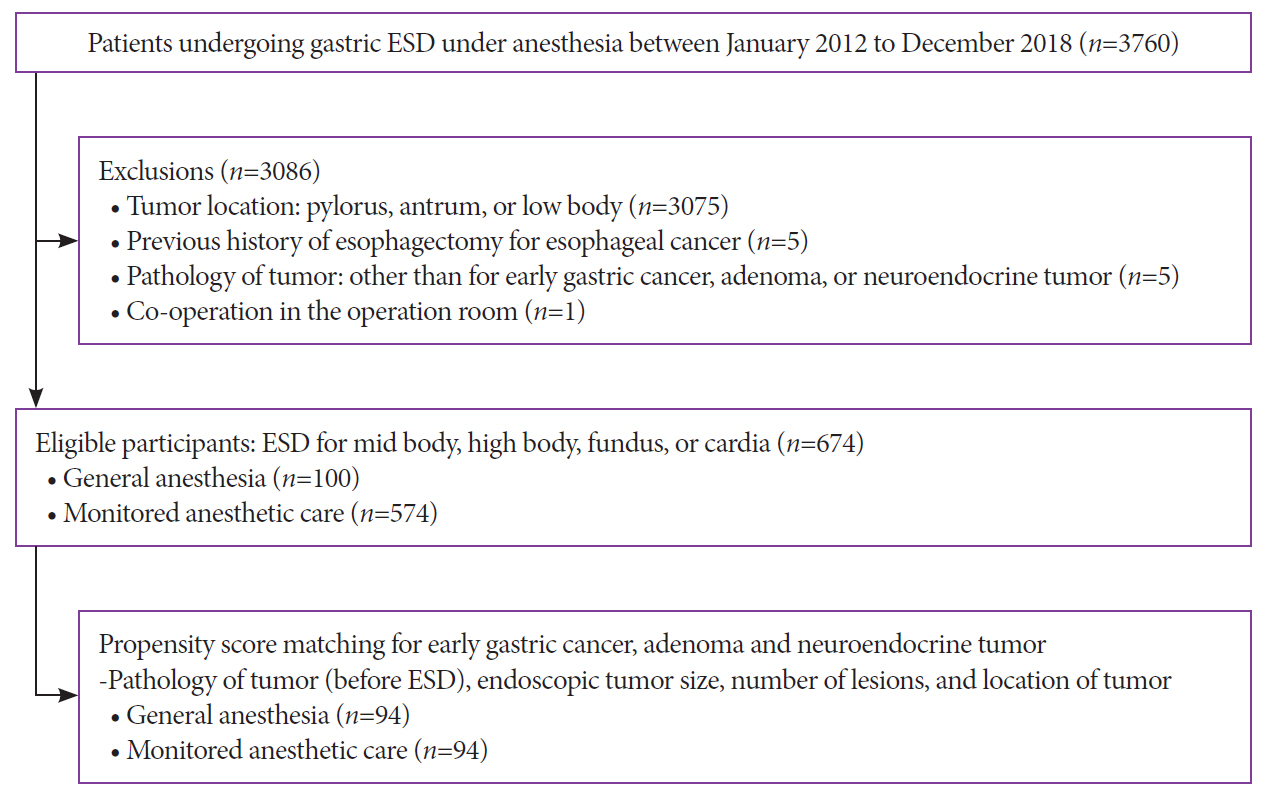
Between 2012 and 2018, 674 patients underwent ESD for gastric tumors in the midbody, high body, fundus, or cardia (100 patients received GA; 574 received MAC). The outcomes of the propensity score (PS)-matched (1:1) patients receiving either GA or MAC were analyzed.
Results
The PS matching identified 94 patients who received GA and 94 patients who received MAC. Both groups showed high rates of en bloc resection (GA, 95.7%; MAC, 97.9%; p=0.68) and complete resection (GA, 81.9%; MAC, 84.0%; p=0.14). There were no significant differences between the rates of adverse events (GA, 16.0%; MAC, 8.5%; p=0.18) in the anesthetic groups. Logistic regression analysis indicated that the method of anesthesia did not affect the rates of complete resection or adverse events.
Conclusions
ESD of tumors in the mid-to-upper stomach at our high-volume center had good outcomes, regardless of the method of anesthesia. Our results demonstrate no differences between the efficacies and safety of ESD performed under MAC and GA.
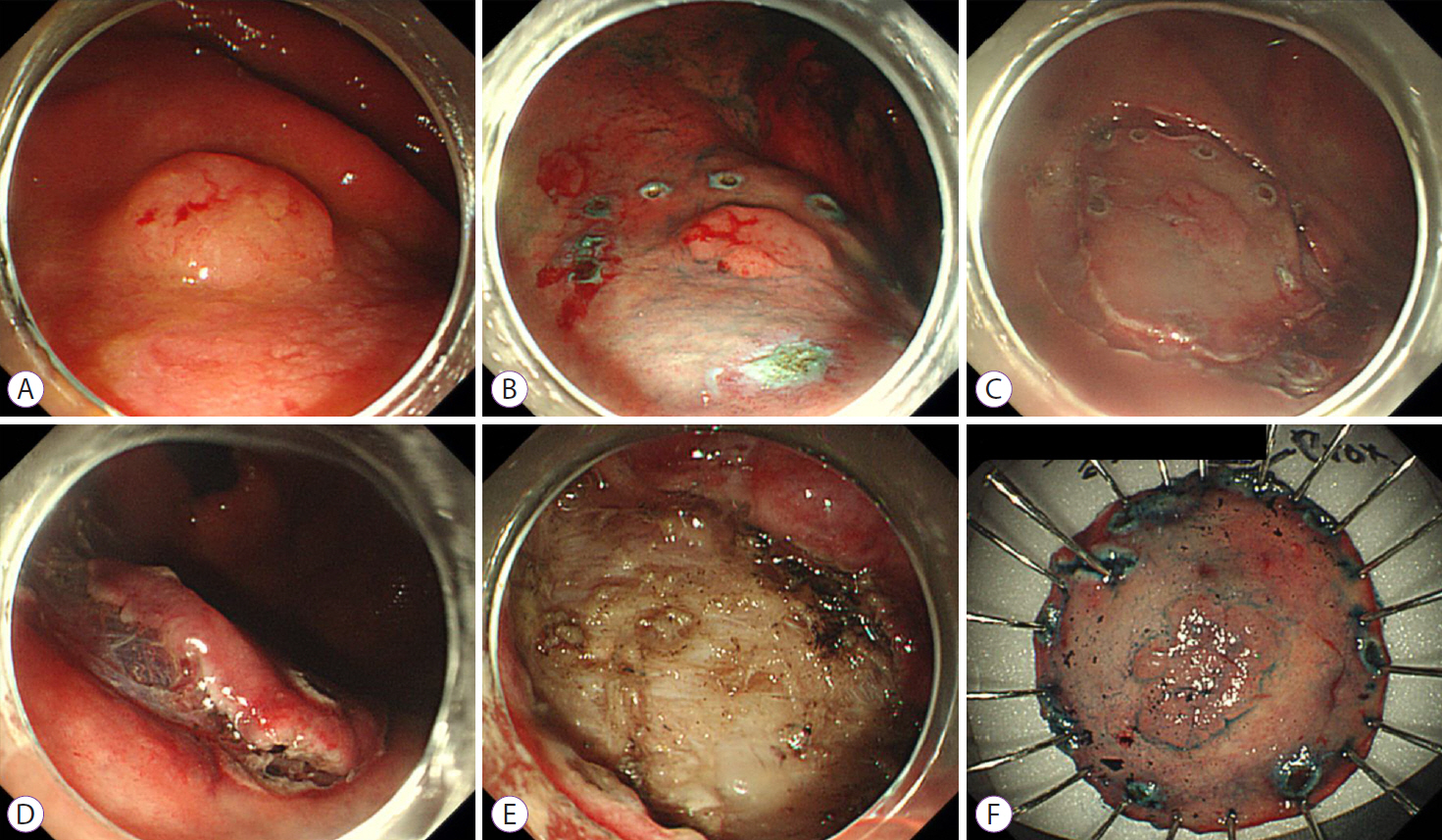
Figure
Reference
-
1. Yurtlu DA, Aslan F, Ayvat P, et al. Propofol-based sedation versus general anesthesia for endoscopic submucosal dissection. Medicine (Baltimore). 2016; 95:e3680.2. Nonaka S, Kawaguchi Y, Oda I, et al. Safety and effectiveness of propofol-based monitored anesthesia care without intubation during endoscopic submucosal dissection for early gastric and esophageal cancers. Dig Endosc. 2015; 27:665–673.3. Kim JH, Nam HS, Choi CW, et al. Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc. 2017; 31:1617–1626.4. Nishiyama N, Kobara H, Fujihara S, et al. Endoscopic submucosal dissection for neoplasia of the greater curvature of the upper and middle stomach: j-shaped superficial cutting and splashed dissection. J Gastrointestin Liver Dis. 2019; 28:397–404.5. Kang DH, Choi CW, Kim HW, et al. Location characteristics of early gastric cancer treated with endoscopic submucosal dissection. Surg Endosc. 2017; 31:4673–4679.6. Kiriyama S, Gotoda T, Sano H, et al. Safe and effective sedation in endoscopic submucosal dissection for early gastric cancer: a randomized comparison between propofol continuous infusion and intermittent midazolam injection. J Gastroenterol. 2010; 45:831–837.7. Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017; 15:194–206.8. Park CH, Shin S, Lee SK, et al. Assessing the stability and safety of procedure during endoscopic submucosal dissection according to sedation methods: a randomized trial. PLoS One. 2015; 10:e0120529.9. Yamaguchi D, Yamaguchi N, Takeuchi Y, et al. Comparison of sedation between the endoscopy room and operation room during endoscopic submucosal dissection for neoplasms in the upper gastrointestinal tract. BMC Gastroenterol. 2017; 17:127.10. Yamashita K, Shiwaku H, Ohmiya T, et al. Efficacy and safety of endoscopic submucosal dissection under general anesthesia. World J Gastrointest Endosc. 2016; 8:466–471.11. Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: a meta-analysis. World J Gastrointest Endosc. 2014; 6:555–563.12. Choi IJ, Lee NR, Kim SG, et al. Short-term outcomes of endoscopic submucosal dissection in patients with early gastric cancer: a prospective multicenter cohort study. Gut Liver. 2016; 10:739–748.13. Yoo JH, Shin SJ, Lee KM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012; 26:2456–2464.14. Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005; 17:54–58.15. Mannen K, Tsunada S, Hara M, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010; 45:30–36.16. Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012; 27:907–912.17. Song BG, Min YW, Cha RR, et al. Endoscopic submucosal dissection under general anesthesia for superficial esophageal squamous cell carcinoma is associated with better clinical outcomes. BMC Gastroenterol. 2018; 18:80.18. Boulos NM, Burton BN, Carter D, Marmor RA, Gabriel RA. Monitored anesthesia care is associated with a decrease in morbidity after endovascular angioplasty in aortoiliac disease. J Cardiothorac Vasc Anesth. 2020; 34:2440–2445.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perforation of a Gastric Tear during Esophageal Endoscopic Submucosal Dissection under General Anesthesia
- Papillary Adenocarcinoma
- Is General Anesthesia Needed in Endoscopic Submucosal Dissection for Lesions Located in the Mid to Upper Stomach?
- Severe respiratory depression precipitated by unrecognized gastric perforation during endoscopic submucosal dissection under deep sedation: A case report
- The Clinical Accuracy of Endoscopic Ultrasonography and White Light Imaging in Gastric Endoscopic Submucosal Dissection