Korean J Physiol Pharmacol.
2022 Jan;26(1):25-36. 10.4196/kjpp.2022.26.1.25.
Carbon monoxide activation of delayed rectifier potassium currents of human cardiac fibroblasts through diverse pathways
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chung-Ang University, Seoul 06974,
- 2Department of Internal Medicine, College of Medicine, Chung-Ang University Hospital, Seoul 06973, Korea
- KMID: 2524517
- DOI: http://doi.org/10.4196/kjpp.2022.26.1.25
Abstract
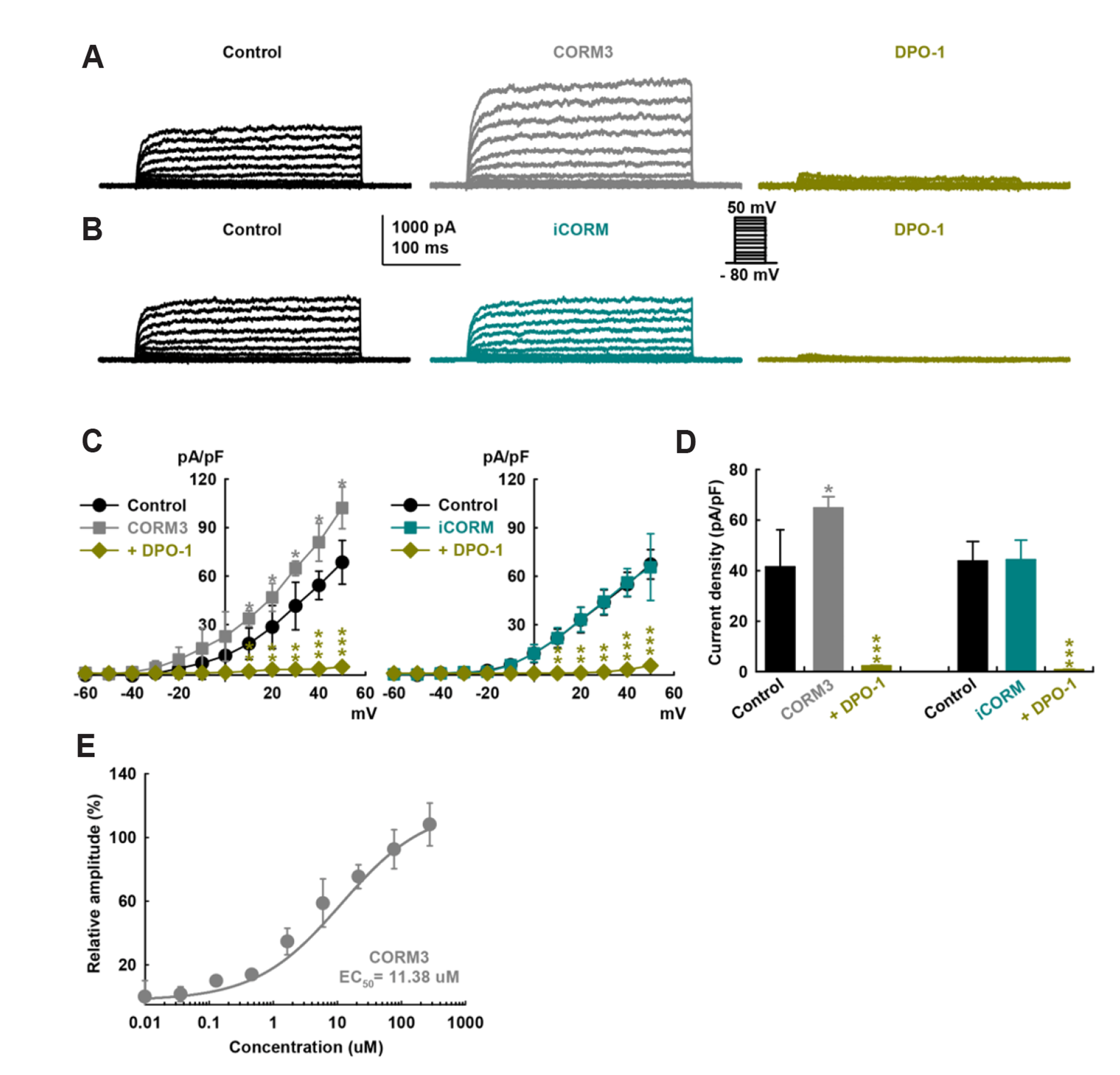
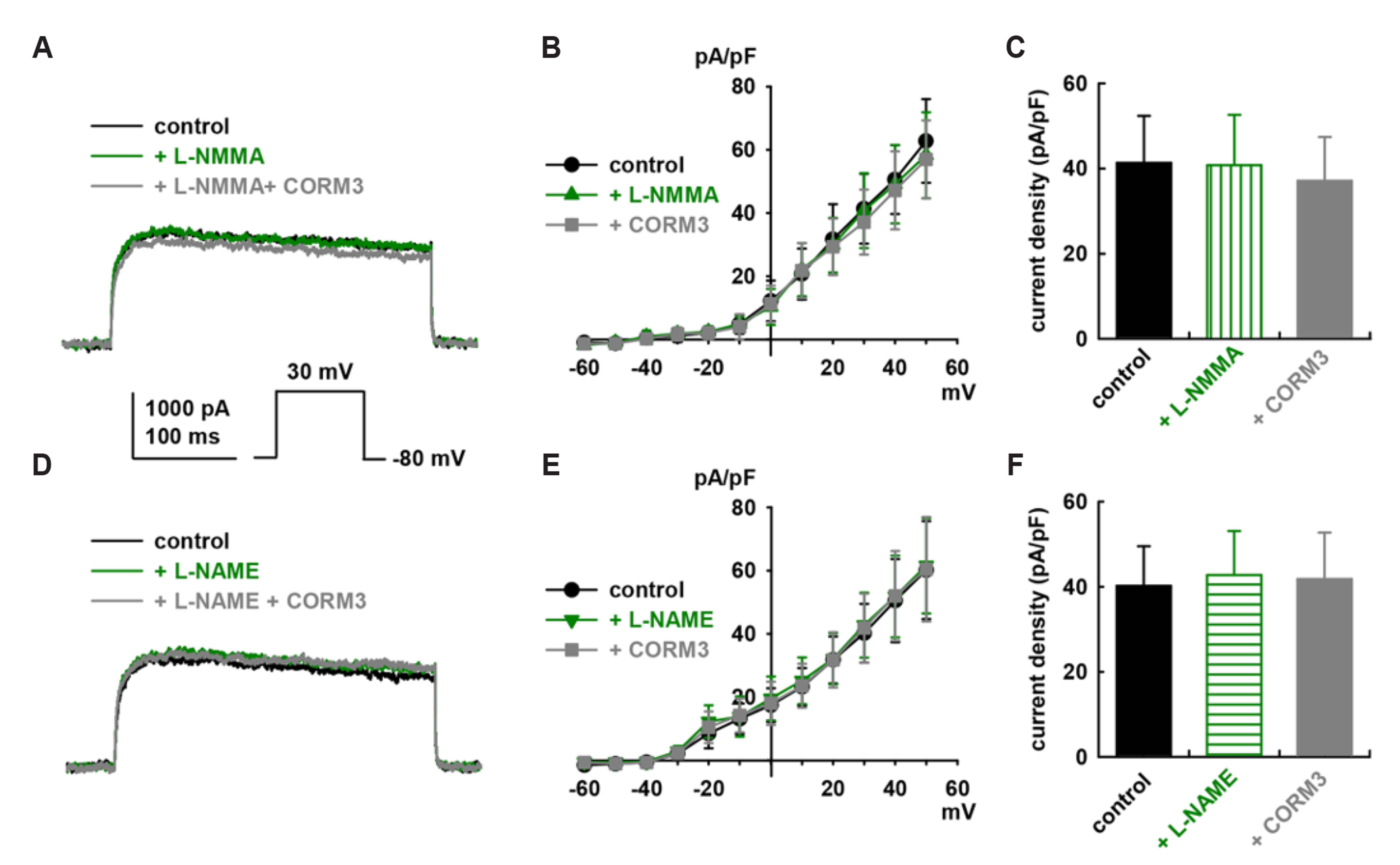
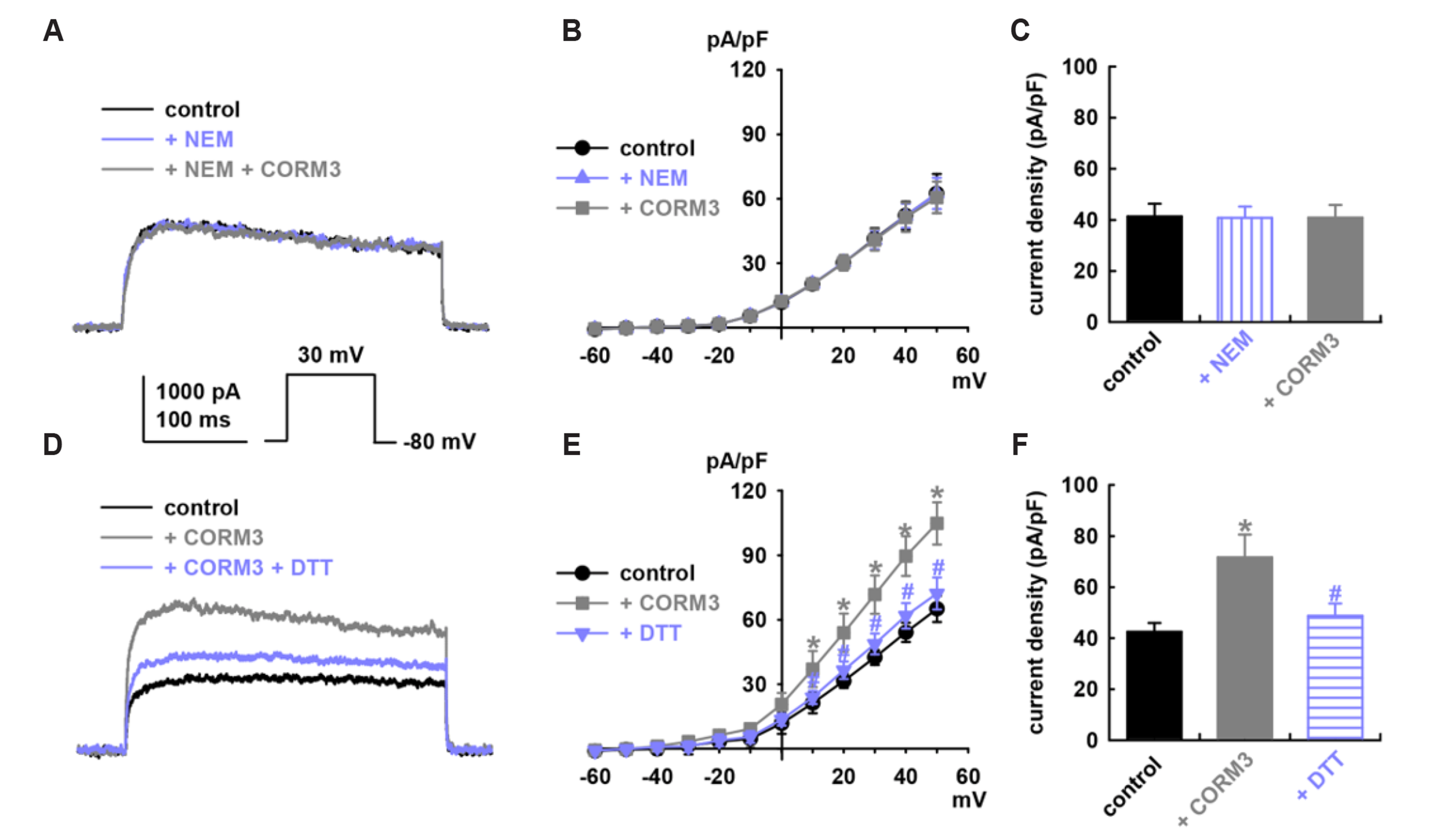
- To identify the effect and mechanism of carbon monoxide (CO) on delayed rectifier K+ currents (IK) of human cardiac fibroblasts (HCFs), we used the wholecell mode patch-clamp technique. Application of CO delivered by carbon monoxidereleasing molecule-3 (CORM3) increased the amplitude of outward K+ currents, and diphenyl phosphine oxide-1 (a specific IK blocker) inhibited the currents. CORM3-induced augmentation was blocked by pretreatment with nitric oxide synthase blockers (L-NG-monomethyl arginine citrate and L-NG-nitro arginine methyl ester). Pretreatment with KT5823 (a protein kinas G blocker), 1H-[1,-2,-4] oxadiazolo-[4,-3-a] quinoxalin-1-on (ODQ, a soluble guanylate cyclase blocker), KT5720 (a protein kinase A blocker), and SQ22536 (an adenylate cyclase blocker) blocked the CORM3 stimulating effect on IK . In addition, pretreatment with SB239063 (a p38 mitogen-activated protein kinase [MAPK] blocker) and PD98059 (a p44/42 MAPK blocker) also blocked the CORM3’s effect on the currents. When testing the involvement of S-nitrosylation, pretreatment of N-ethylmaleimide (a thiol-alkylating reagent) blocked CO-induced IK activation and DL-dithiothreitol (a reducing agent) reversed this effect. Pretreatment with 5,10,15,20-tetrakis(1-methylpyridinium-4-yl)-21H,23H porphyrin manganese (III) pentachloride and manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (superoxide dismutase mimetics), diphenyleneiodonium chloride (an NADPH oxidase blocker), or allopurinol (a xanthine oxidase blocker) also inhibited CO-induced IK activation. These results suggest that CO enhances IK in HCFs through the nitric oxide, phosphorylation by protein kinase G, protein kinase A, and MAPK, S-nitrosylation and reduction/oxidation (redox) signaling pathways.
Figure
Reference
-
1. Ernst A, Zibrak JD. 1998; Carbon monoxide poisoning. N Engl J Med. 339:1603–1608. DOI: 10.1056/NEJM199811263392206. PMID: 9828249.
Article2. Ewing JF, Raju VS, Maines MD. 1994; Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3':5'-guanosine monophosphate. J Pharmacol Exp Ther. 271:408–414. PMID: 7525927.3. Lakkisto P, Palojoki E, Bäcklund T, Saraste A, Tikkanen I, Voipio-Pulkki LM, Pulkki K. 2002; Expression of heme oxygenase-1 in response to myocardial infarction in rats. J Mol Cell Cardiol. 34:1357–1365. DOI: 10.1006/jmcc.2002.2094. PMID: 12392996.
Article4. Shan H, Li T, Zhang L, Yang R, Li Y, Zhang M, Dong Y, Zhou Y, Xu C, Yang B, Liang H, Gao X, Shan H. 2019; Heme oxygenase-1 prevents heart against myocardial infarction by attenuating ischemic injury-induced cardiomyocytes senescence. EBioMedicine. 39:59–68. DOI: 10.1016/j.ebiom.2018.11.056. PMID: 30527623. PMCID: PMC6355645.
Article5. Ndisang JF, Chibbar R, Lane N. 2014; Heme oxygenase suppresses markers of heart failure and ameliorates cardiomyopathy in L-NAME-induced hypertension. Eur J Pharmacol. 734:23–34. DOI: 10.1016/j.ejphar.2014.03.026. PMID: 24726875.
Article6. Cheng Y, Rong J. 2017; Therapeutic potential of heme oxygenase-1/carbon monoxide system against ischemia-reperfusion injury. Curr Pharm Des. 23:3884–3898. DOI: 10.2174/1381612823666170413122439. PMID: 28412905.
Article7. Motterlini R, Otterbein LE. 2010; The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 9:728–743. DOI: 10.1038/nrd3228. PMID: 20811383.
Article8. Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. 2009; Dual pathways of carbon monoxide-mediated vasoregulation: modulation by redox mechanisms. Circ Res. 105:775–783. DOI: 10.1161/CIRCRESAHA.109.197434. PMID: 19745167. PMCID: PMC2771695.9. Motterlini R. 2007; Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans. 35(Pt 5):1142–1146. DOI: 10.1042/BST0351142. PMID: 17956297.
Article10. Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. 2004; Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 18:771–772. DOI: 10.1096/fj.03-0921fje. PMID: 14977880.11. Dallas ML, Boyle JP, Milligan CJ, Sayer R, Kerrigan TL, McKinstry C, Lu P, Mankouri J, Harris M, Scragg JL, Pearson HA, Peers C. 2011; Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J. 25:1519–1530. DOI: 10.1096/fj.10-173450. PMID: 21248240.12. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. 2000; Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 6:422–428. DOI: 10.1038/74680. PMID: 10742149.
Article13. Li L, Hsu A, Moore PK. 2009; Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation--a tale of three gases! Pharmacol Ther. 123:386–400. DOI: 10.1016/j.pharmthera.2009.05.005. PMID: 19486912.14. Grandi E, Sanguinetti MC, Bartos DC, Bers DM, Chen-Izu Y, Chiamvimonvat N, Colecraft HM, Delisle BP, Heijman J, Navedo MF, Noskov S, Proenza C, Vandenberg JI, Yarov-Yarovoy V. 2017; Potassium channels in the heart: structure, function and regulation. J Physiol. 595:2209–2228. DOI: 10.1113/JP272864. PMID: 27861921. PMCID: PMC5374109.
Article15. Chen L, Sampson KJ, Kass RS. 2016; Cardiac delayed rectifier potassium channels in health and disease. Card Electrophysiol Clin. 8:307–322. DOI: 10.1016/j.ccep.2016.01.004. PMID: 27261823. PMCID: PMC4893812.
Article16. Wang R, Wang Z, Wu L. 1997; Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 121:927–934. DOI: 10.1038/sj.bjp.0701222. PMID: 9222549. PMCID: PMC1564776.
Article17. Dallas ML, Scragg JL, Peers C. 2008; Modulation of hTREK-1 by carbon monoxide. Neuroreport. 19:345–348. DOI: 10.1097/WNR.0b013e3282f51045. PMID: 18303579.
Article18. Liang S, Wang Q, Zhang W, Zhang H, Tan S, Ahmed A, Gu Y. 2014; Carbon monoxide inhibits inward rectifier potassium channels in cardiomyocytes. Nat Commun. 5:4676. DOI: 10.1038/ncomms5676. PMID: 25118981.
Article19. Althaus M, Fronius M, Buchäckert Y, Vadász I, Clauss WG, Seeger W, Motterlini R, Morty RE. 2009; Carbon monoxide rapidly impairs alveolar fluid clearance by inhibiting epithelial sodium channels. Am J Respir Cell Mol Biol. 41:639–650. DOI: 10.1165/rcmb.2008-0458OC. PMID: 19251942.
Article20. Wang S, Publicover S, Gu Y. 2009; An oxygen-sensitive mechanism in regulation of epithelial sodium channel. Proc Natl Acad Sci U S A. 106:2957–2962. DOI: 10.1073/pnas.0809100106. PMID: 19196957. PMCID: PMC2650320.
Article21. Dallas ML, Scragg JL, Peers C. 2009; Inhibition of L-type Ca2+ channels by carbon monoxide. Adv Exp Med Biol. 648:89–95. DOI: 10.1007/978-90-481-2259-2_10. PMID: 19536469.22. Lim I, Gibbons SJ, Lyford GL, Miller SM, Strege PR, Sarr MG, Chatterjee S, Szurszewski JH, Shah VH, Farrugia G. 2005; Carbon monoxide activates human intestinal smooth muscle L-type Ca2+ channels through a nitric oxide-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 288:G7–G14. DOI: 10.1152/ajpgi.00205.2004. PMID: 15319183.23. Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C. 2008; Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem. 283:24412–24419. DOI: 10.1074/jbc.M803037200. PMID: 18596041. PMCID: PMC3259849.24. Camelliti P, Borg TK, Kohl P. 2005; Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 65:40–51. DOI: 10.1016/j.cardiores.2004.08.020. PMID: 15621032.
Article25. Krenning G, Zeisberg EM, Kalluri R. 2010; The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 225:631–637. DOI: 10.1002/jcp.22322. PMID: 20635395. PMCID: PMC3098503.
Article26. Vliegen HW, van der Laarse A, Cornelisse CJ, Eulderink F. 1991; Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur Heart J. 12:488–494. DOI: 10.1093/oxfordjournals.eurheartj.a059928. PMID: 1829680.27. Villarreal FJ, Kim NN. 1998; Regulation of myocardial extracellular matrix components by mechanical and chemical growth factors. Cardiovasc Pathol. 7:145–151. DOI: 10.1016/S1054-8807(97)00122-1. PMID: 25851221.
Article28. Camelliti P, Green CR, LeGrice I, Kohl P. 2004; Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 94:828–835. DOI: 10.1161/01.RES.0000122382.19400.14. PMID: 14976125.29. Kohl P. 2003; Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ Res. 93:381–383. DOI: 10.1161/01.RES.0000091364.90121.0C. PMID: 12958139.30. Gaudesius G, Miragoli M, Thomas SP, Rohr S. 2003; Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 93:421–428. DOI: 10.1161/01.RES.0000089258.40661.0C. PMID: 12893743.
Article31. Asada K, Kurokawa J, Furukawa T. 2009; Redox- and calmodulin-dependent S-nitrosylation of the KCNQ1 channel. J Biol Chem. 284:6014–6020. DOI: 10.1074/jbc.M807158200. PMID: 19124472.
Article32. Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. 2009; Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One. 4:e7307. DOI: 10.1371/journal.pone.0007307. PMID: 19806193. PMCID: PMC2751830.
Article33. Yue L, Xie J, Nattel S. 2011; Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 89:744–753. DOI: 10.1093/cvr/cvq329. PMID: 20962103. PMCID: PMC3039247.
Article34. Miragoli M, Salvarani N, Rohr S. 2007; Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 101:755–758. DOI: 10.1161/CIRCRESAHA.107.160549. PMID: 17872460.
Article35. Pellman J, Zhang J, Sheikh F. 2016; Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: mechanisms and model systems. J Mol Cell Cardiol. 94:22–31. DOI: 10.1016/j.yjmcc.2016.03.005. PMID: 26996756. PMCID: PMC4861678.
Article36. Bae H, Lee D, Kim YW, Choi J, Lee HJ, Kim SW, Kim T, Noh YH, Ko JH, Bang H, Lim I. 2016; Effects of hydrogen peroxide on voltage-dependent K+ currents in human cardiac fibroblasts through protein kinase pathways. Korean J Physiol Pharmacol. 20:315–324. DOI: 10.4196/kjpp.2016.20.3.315. PMID: 27162486. PMCID: PMC4860374.37. Bae H, Kim T, Lim I. 2021; Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms. Korean J Physiol Pharmacol. 25:227–237. DOI: 10.4196/kjpp.2021.25.3.227. PMID: 33859063. PMCID: PMC8050612.
Article38. Yang R, Liu Y, Hou X, Fan Y, Li J, Chen M, Wang Y, Zhang X, Zhang M. 2018; MAPKs-mediated modulation of the myocyte voltage-gated K+ channels is involved in ethanol-induced rat coronary arterial contraction. Eur J Pharmacol. 834:274–280. DOI: 10.1016/j.ejphar.2018.07.042. PMID: 30053411.39. Lagrutta A, Wang J, Fermini B, Salata JJ. 2006; Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 317:1054–1063. DOI: 10.1124/jpet.106.101162. PMID: 16522807.40. Wang Z, Fermini B, Nattel S. 1993; Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 73:276–285. DOI: 10.1161/01.RES.73.2.276. PMID: 8330373.
Article41. Wang Z, Fermini B, Nattel S. 1993; Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 73:1061–1076. DOI: 10.1161/01.RES.73.6.1061. PMID: 8222078.42. Abderrahmane A, Salvail D, Dumoulin M, Garon J, Cadieux A, Rousseau E. 1998; Direct activation of KCa channel in airway smooth muscle by nitric oxide: involvement of a nitrothiosylation mechanism? Am J Respir Cell Mol Biol. 19:485–497. DOI: 10.1165/ajrcmb.19.3.2996. PMID: 9730877.43. Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, Nattel S. 2016; The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res. 109:467–479. DOI: 10.1093/cvr/cvv275. PMID: 26705366. PMCID: PMC4777910.
Article44. Ravens U, Wettwer E. 2011; Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc Res. 89:776–785. DOI: 10.1093/cvr/cvq398. PMID: 21159668.
Article45. Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. 2010; Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog Biophys Mol Biol. 103:95–101. DOI: 10.1016/j.pbiomolbio.2009.10.001. PMID: 19835907.46. Feng J, Wible B, Li GR, Wang Z, Nattel S. 1997; Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 80:572–579. DOI: 10.1161/01.RES.80.4.572. PMID: 9118489.47. Núñez L, Vaquero M, Gómez R, Caballero R, Mateos-Cáceres P, Macaya C, Iriepa I, Gálvez E, López-Farré A, Tamargo J, Delpón E. 2006; Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res. 72:80–89. DOI: 10.1016/j.cardiores.2006.06.021. PMID: 16876149.48. Schmitt N, Grunnet M, Olesen SP. 2014; Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 94:609–653. DOI: 10.1152/physrev.00022.2013. PMID: 24692356.
Article49. Wettwer E, Terlau H. 2014; Pharmacology of voltage-gated potassium channel Kv1.5--impact on cardiac excitability. Curr Opin Pharmacol. 15:115–121. DOI: 10.1016/j.coph.2014.02.001. PMID: 24632326.
Article50. Ravens U, Odening KE. 2017; Atrial fibrillation: therapeutic potential of atrial K+ channel blockers. Pharmacol Ther. 176:13–21. DOI: 10.1016/j.pharmthera.2016.10.003. PMID: 27742566.51. Bae H, Choi J, Kim YW, Lee D, Kim JH, Ko JH, Bang H, Kim T, Lim I. 2018; Effects of nitric oxide on voltage-gated K+ currents in human cardiac fibroblasts through the protein kinase G and protein kinase A pathways but not through S-nitrosylation. Int J Mol Sci. 19:814. DOI: 10.3390/ijms19030814. PMID: 29534509. PMCID: PMC5877675.52. Ordög B, Brutyó E, Puskás LG, Papp JG, Varró A, Szabad J, Boldogkoi Z. 2006; Gene expression profiling of human cardiac potassium and sodium channels. Int J Cardiol. 111:386–393. DOI: 10.1016/j.ijcard.2005.07.063. PMID: 16257073.53. Al-Owais MM, Hettiarachchi NT, Boyle JP, Scragg JL, Elies J, Dallas ML, Lippiat JD, Steele DS, Peers C. 2017; Multiple mechanisms mediating carbon monoxide inhibition of the voltage-gated K+ channel Kv1.5. Cell Death Dis. 8:e3163. DOI: 10.1038/cddis.2017.568. PMID: 29095440. PMCID: PMC5775415.54. Hartsfield CL. 2002; Cross talk between carbon monoxide and nitric oxide. Antioxid Redox Signal. 4:301–307. DOI: 10.1089/152308602753666352. PMID: 12006181.
Article55. Ingi T, Cheng J, Ronnett GV. 1996; Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 16:835–842. DOI: 10.1016/S0896-6273(00)80103-8. PMID: 8608001.
Article56. Durante W, Christodoulides N, Cheng K, Peyton KJ, Sunahara RK, Schafer AI. 1997; cAMP induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle. Am J Physiol. 273(1 Pt 2):H317–H323. DOI: 10.1152/ajpheart.1997.273.1.H317. PMID: 9249506.
Article57. Polte T, Abate A, Dennery PA, Schröder H. 2000; Heme oxygenase-1 is a cGMP-inducible endothelial protein and mediates the cytoprotective action of nitric oxide. Arterioscler Thromb Vasc Biol. 20:1209–1215. DOI: 10.1161/01.ATV.20.5.1209. PMID: 10807735.
Article58. Bai CX, Takahashi K, Masumiya H, Sawanobori T, Furukawa T. 2004; Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes. Br J Pharmacol. 142:567–575. DOI: 10.1038/sj.bjp.0705814. PMID: 15148247. PMCID: PMC1574975.59. Shimizu K, Shintani Y, Ding WG, Matsuura H, Bamba T. 2002; Potentiation of slow component of delayed rectifier K+ current by cGMP via two distinct mechanisms: inhibition of phosphodiesterase 3 and activation of protein kinase G. Br J Pharmacol. 137:127–137. DOI: 10.1038/sj.bjp.0704843. PMID: 12183338. PMCID: PMC1573469.60. Brüne B, Ullrich V. 1987; Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 32:497–504. PMID: 2890093.61. Kharitonov VG, Sharma VS, Pilz RB, Magde D, Koesling D. 1995; Basis of guanylate cyclase activation by carbon monoxide. Proc Natl Acad Sci U S A. 92:2568–2571. DOI: 10.1073/pnas.92.7.2568. PMID: 7708686. PMCID: PMC42259.
Article62. Zhang YH. 2017; Nitric oxide signalling and neuronal nitric oxide synthase in the heart under stress. F1000Res. 6:742. DOI: 10.12688/f1000research.10128.1. PMID: 28649367. PMCID: PMC5464233.
Article63. Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. 1999; Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 84:1020–1031. DOI: 10.1161/01.RES.84.9.1020. PMID: 10325239.
Article64. Abramochkin DV, Konovalova OP, Kamkin A, Sitdikova GF. 2015; Carbon monoxide modulates electrical activity of murine myocardium via cGMP-dependent mechanisms. J Physiol Biochem. 71:107–119. DOI: 10.1007/s13105-015-0387-y. PMID: 25670496.
Article65. Dallas ML, Yang Z, Boyle JP, Boycott HE, Scragg JL, Milligan CJ, Elies J, Duke A, Thireau J, Reboul C, Richard S, Bernus O, Steele DS, Peers C. 2012; Carbon monoxide induces cardiac arrhythmia via induction of the late Na+ current. Am J Respir Crit Care Med. 186:648–656. DOI: 10.1164/rccm.201204-0688OC. PMID: 22822026. PMCID: PMC3622900.66. Bae H, Lim I. 2017; Effects of nitric oxide on large-conductance Ca2+-activated K+ currents in human cardiac fibroblasts through PKA and PKG-related pathways. Clin Exp Pharmacol Physiol. 44:1116–1124. DOI: 10.1111/1440-1681.12817. PMID: 28731589.67. Kim HP, Ryter SW, Choi AM. 2006; CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 46:411–449. DOI: 10.1146/annurev.pharmtox.46.120604.141053. PMID: 16402911.
Article68. Ryter SW, Alam J, Choi AM. 2006; Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 86:583–650. DOI: 10.1152/physrev.00011.2005. PMID: 16601269.
Article69. Ishii T, Warabi E, Siow RCM, Mann GE. 2013; Sequestosome1/p62: a regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radic Biol Med. 65:102–116. DOI: 10.1016/j.freeradbiomed.2013.06.019. PMID: 23792273.
Article70. Boczkowski J, Poderoso JJ, Motterlini R. 2006; CO-metal interaction: vital signaling from a lethal gas. Trends Biochem Sci. 31:614–621. DOI: 10.1016/j.tibs.2006.09.001. PMID: 16996273.
Article71. André L, Gouzi F, Thireau J, Meyer G, Boissiere J, Delage M, Abdellaoui A, Feillet-Coudray C, Fouret G, Cristol JP, Lacampagne A, Obert P, Reboul C, Fauconnier J, Hayot M, Richard S, Cazorla O. 2011; Carbon monoxide exposure enhances arrhythmia after cardiac stress: involvement of oxidative stress. Basic Res Cardiol. 106:1235–1246. DOI: 10.1007/s00395-011-0211-y. PMID: 21822772.
Article72. Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marbán E, Hare JM. 2001; Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 104:2407–2411. DOI: 10.1161/hc4501.098928. PMID: 11705816.
Article73. Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. 2005; Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 115:1221–1231. DOI: 10.1172/JCI21968. PMID: 15841206. PMCID: PMC1077169.
Article74. Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. 2003; Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 41:2164–2171. DOI: 10.1016/S0735-1097(03)00471-6. PMID: 12821241.
Article75. Nabeebaccus A, Zhang M, Shah AM. 2011; NADPH oxidases and cardiac remodelling. Heart Fail Rev. 16:5–12. DOI: 10.1007/s10741-010-9186-2. PMID: 20658317.
Article76. Iwata K, Matsuno K, Murata A, Zhu K, Fukui H, Ikuta K, Katsuyama M, Ibi M, Matsumoto M, Ohigashi M, Wen X, Zhang J, Cui W, Yabe-Nishimura C. 2018; Up-regulation of NOX1/NADPH oxidase following drug-induced myocardial injury promotes cardiac dysfunction and fibrosis. Free Radic Biol Med. 120:277–288. DOI: 10.1016/j.freeradbiomed.2018.03.053. PMID: 29609020.
Article77. Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, Weissmann N. 2012; Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med. 52:1033–1042. DOI: 10.1016/j.freeradbiomed.2011.12.004. PMID: 22222468.
Article78. Corradi D, Callegari S, Maestri R, Benussi S, Bosio S, De Palma G, Alinovi R, Caglieri A, Goldoni M, Mozzoni P, Pastori P, Manotti L, Nascimbene S, Dorigo E, Rusconi R, Astorri E, Alfieri O. 2008; Heme oxygenase-1 expression in the left atrial myocardium of patients with chronic atrial fibrillation related to mitral valve disease: its regional relationship with structural remodeling. Hum Pathol. 39:1162–1171. DOI: 10.1016/j.humpath.2007.12.007. PMID: 18440590.
Article79. Yeh YH, Hsu LA, Chen YH, Kuo CT, Chang GJ, Chen WJ. 2016; Protective role of heme oxygenase-1 in atrial remodeling. Basic Res Cardiol. 111:58. DOI: 10.1007/s00395-016-0577-y. PMID: 27562817.
Article80. Leffler CW, Parfenova H, Jaggar JH, Wang R. 2006; Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol (1985). 100:1065–1076. DOI: 10.1152/japplphysiol.00793.2005. PMID: 16467393. PMCID: PMC1363746.
Article81. Durante W, Johnson FK, Johnson RA. 2006; Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 10:672–686. DOI: 10.1111/j.1582-4934.2006.tb00427.x. PMID: 16989727. PMCID: PMC3933149.
Article82. Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. 1997; Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 80:557–564. DOI: 10.1161/01.RES.80.4.557. PMID: 9118487.
Article83. Otterbein LE, Foresti R, Motterlini R. 2016; Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res. 118:1940–1959. DOI: 10.1161/CIRCRESAHA.116.306588. PMID: 27283533. PMCID: PMC4905590.84. Johnson FK, Johnson RA. 2003; Carbon monoxide promotes endothelium-dependent constriction of isolated gracilis muscle arterioles. Am J Physiol Regul Integr Comp Physiol. 285:R536–R541. DOI: 10.1152/ajpregu.00624.2002. PMID: 12676757.85. Lee TS, Chau LY. 2002; Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 8:240–246. DOI: 10.1038/nm0302-240. PMID: 11875494.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chorea as a Clinical Manifestation of Delayed Neurologic Sequelae in Carbon Monoxide Poisoning: Case Report
- Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms
- A Clinical Observation of Acute Carbon Monoxide Poisoning
- Two Components of Voltage Dependent Outward K+ Current in Isolated Human Atrial Myocytes
- A study of neurologic sequelae in carbon monoxide intoxication