J Pathol Transl Med.
2022 Jan;56(1):1-15. 10.4132/jptm.2021.09.29.
Follicular lymphoma: updates for pathologists
- Affiliations
-
- 1Department of Pathology and Hematopathology, St. Jude Children's Research Hospital, Memphis, TN, USA
- 2Department of Pathology, Division of Hematopathology, University of Miami, Sylvester Comprehensive Cancer Center, and Jackson Memorial Hospitals, Miami, FL, USA
- KMID: 2524224
- DOI: http://doi.org/10.4132/jptm.2021.09.29
Abstract
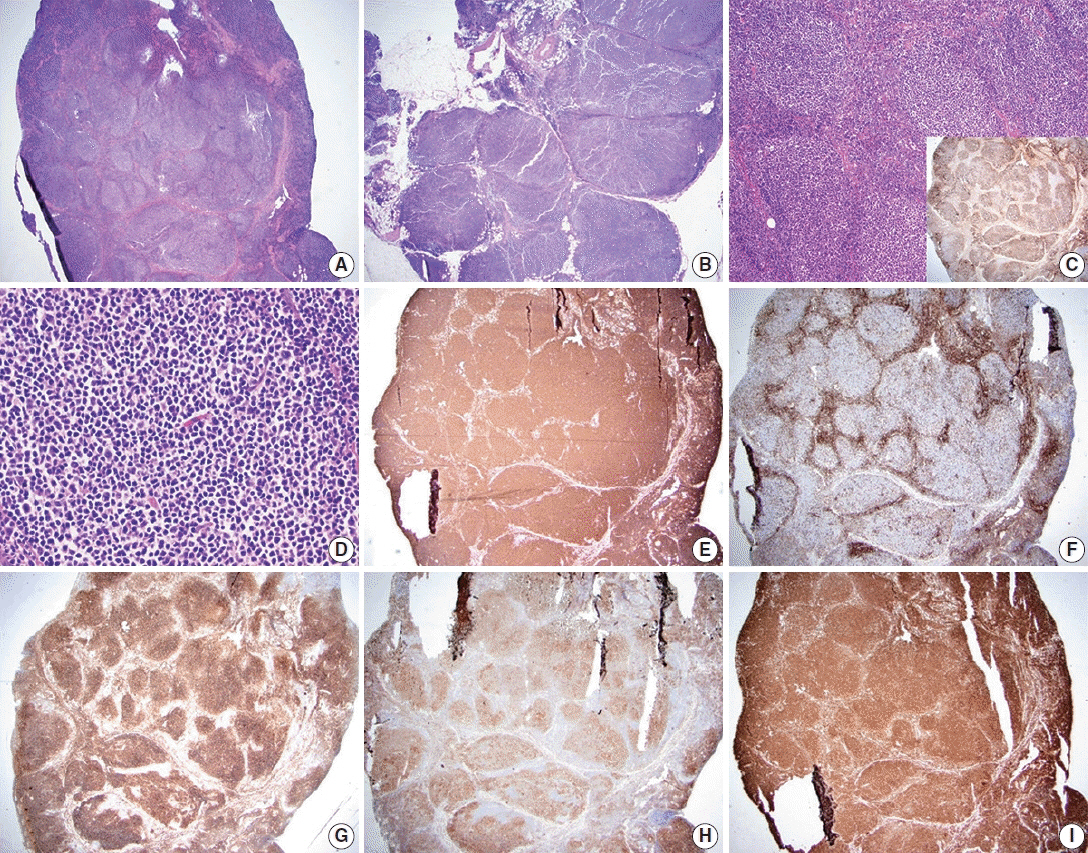
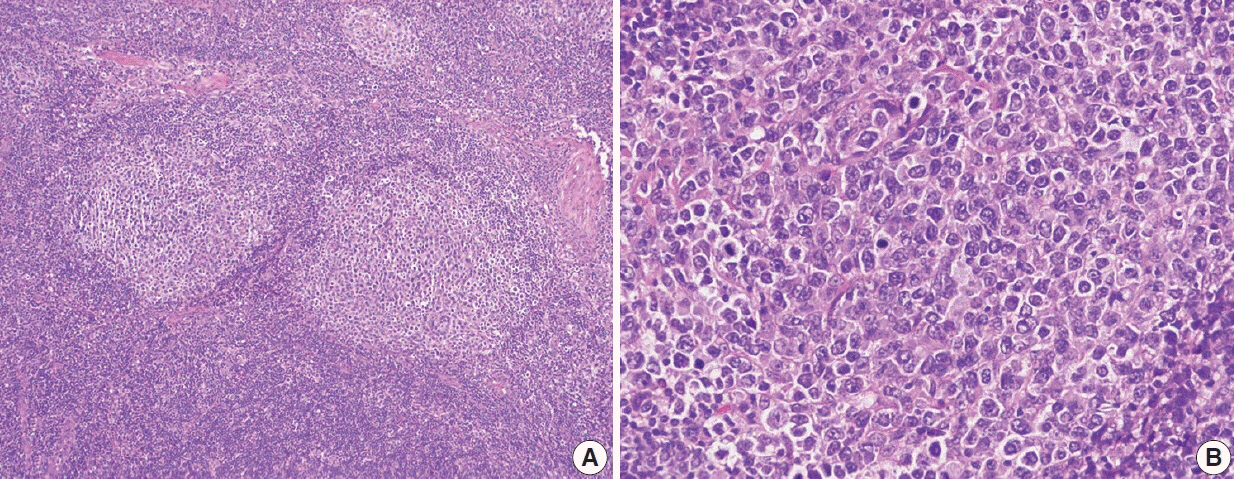
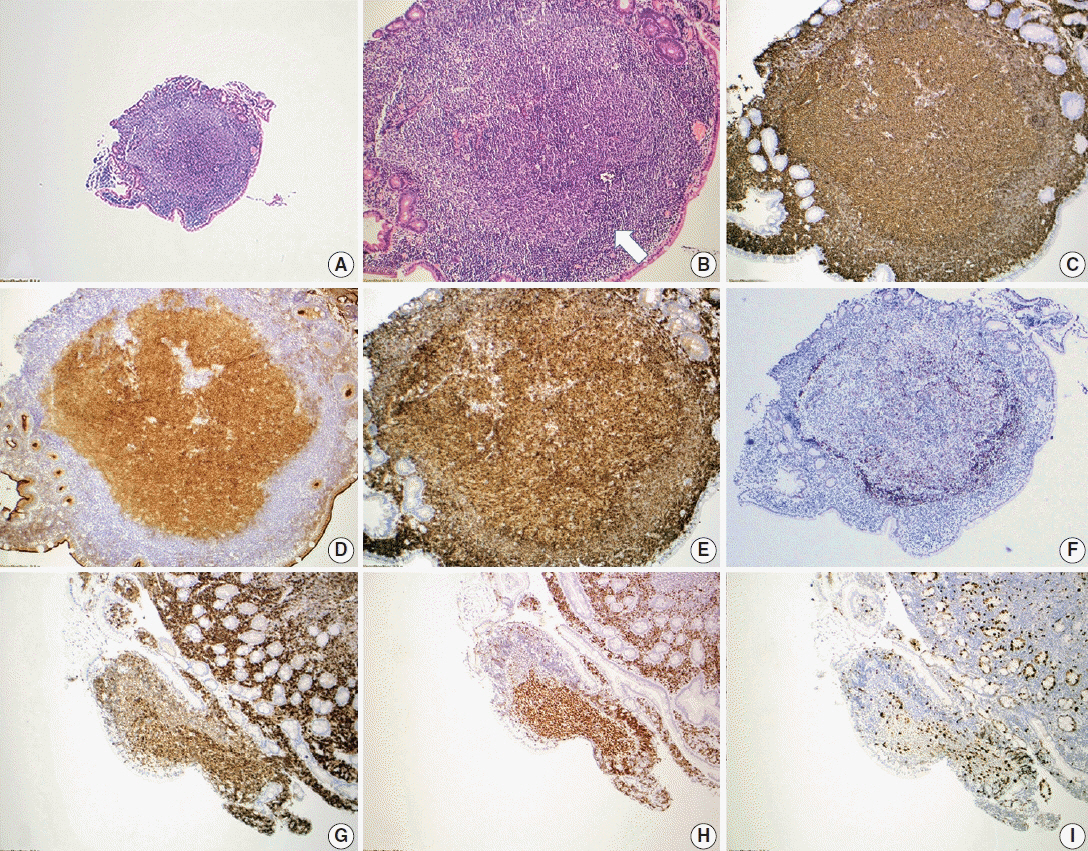
- Follicular lymphoma (FL) is the most common indolent B-cell lymphoma and originates from germinal center B-cells (centrocytes and centroblasts) of the lymphoid follicle. Tumorigenesis is believed to initiate early in precursor B-cells in the bone marrow (BM) that acquire the t(14;18)(q32;q21). These cells later migrate to lymph nodes to continue their maturation through the germinal center reaction, at which time they acquire additional genetic and epigeneticabnormalities that promote lymphomagenesis. FLs are heterogeneous in terms of their clinicopathologic features. Most FLs are indolent and clinically characterized by peripheral lymphadenopathy with involvement of the spleen, BM, and peripheral blood in a substantial subset of patients, sometimes accompanied by constitutional symptoms and laboratory abnormalities. Diagnosis is established by the histopathologic identification of a B-cell proliferation usually distributed in an at least partially follicular pattern, typically, but not always, in a lymph node biopsy. The B-cell proliferation is biologically of germinal center cell origin, thus shows an expression of germinal center-associated antigens as detected by immunophenotyping. Although many cases of FLs are typical and histopathologic features are straightforward, the biologic and histopathologic variability of FL is wide, and an accurate diagnosis of FL over this disease spectrum requires knowledge of morphologic variants that can mimic other lymphomas, and rarely non-hematologic malignancies, clinically unique variants, and pitfalls in the interpretation of ancillary studies. The overall survival for most patients is prolonged, but relapses are frequent. The treatment landscape in FL now includes the application of immunotherapy and targeted therapy in addition to chemotherapy.
Figure
Reference
-
References
1. Jaffe ES, Harris NL, Swerdlow SH, et al. Follicular lymphoma. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2017. p. 266–77.2. Brill NE, Baehr G, Rosenthal N. Generalized giant lymph follicle hyperplasia of lymph nodes and spleen; a hitherto undescribed type. Am J Med. 1952; 13:570–4.3. Lukes RJ, Butler JJ. The pathology and nomenclature of Hodgkin's disease. Cancer Res. 1966; 26:1063–83.4. Lennert K. History of the European Association for Haematopathology. New York: Springer;2006.5. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994; 84:1361–92.6. Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998; 9:717–20.7. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997; 89:3909–18.8. Roulland S, Faroudi M, Mamessier E, Sungalee S, Salles G, Nadel B. Early steps of follicular lymphoma pathogenesis. Adv Immunol. 2011; 111:1–46.
Article9. Sungalee S, Mamessier E, Morgado E, et al. Germinal center reentries of BCL2-overexpressing B cells drive follicular lymphoma progression. J Clin Invest. 2014; 124:5337–51.
Article10. Carbone A, Roulland S, Gloghini A, et al. Follicular lymphoma. Nat Rev Dis Primers. 2019; 5:83.
Article11. Green MR. Chromatin modifying gene mutations in follicular lymphoma. Blood. 2018; 131:595–604.
Article12. Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A. 2015; 112:E1116–25.
Article13. Green MR, Gentles AJ, Nair RV, et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood. 2013; 121:1604–11.
Article14. Mamessier E, Broussais-Guillaumot F, Chetaille B, et al. Nature and importance of follicular lymphoma precursors. Haematologica. 2014; 99:802–10.
Article15. Goldin LR, Bjorkholm M, Kristinsson SY, Turesson I, Landgren O. Highly increased familial risks for specific lymphoma subtypes. Br J Haematol. 2009; 146:91–4.
Article16. Skibola CF, Bracci PM, Halperin E, et al. Genetic variants at 6p21.33 are associated with susceptibility to follicular lymphoma. Nat Genet. 2009; 41:873–5.
Article17. Smith S. Transformed lymphoma: what should I do now? Hematology Am Soc Hematol Educ Program. 2020; 2020:306–11.
Article18. Trecourt A, Mauduit C, Szablewski V, et al. Plasticity of mature B cells between follicular and classic Hodgkin lymphomas: a series of 22 cases expanding the spectrum of transdifferentiation. Am J Surg Pathol. 2022; 46:58–70.19. Sarkozy C, Baseggio L, Feugier P, et al. Peripheral blood involvement in patients with follicular lymphoma: a rare disease manifestation associated with poor prognosis. Br J Haematol. 2014; 164:659–67.
Article20. Yoo C, Yoon DH, Suh C. Serum beta-2 microglobulin in malignant lymphomas: an old but powerful prognostic factor. Blood Res. 2014; 49:148–53.
Article21. Bain BJ. Diagnosis of follicular lymphoma from the peripheral blood. Am J Hematol. 2018; 93:1111–2.
Article22. Medeiros LJ, O'Malley DP, Caraway NP, Vega F, Elenitoba-Johnson KS, Lim MS. AFIP atlas of tumor pathology, series 4. Tumors of the lymph nodes and spleen. Follicular lymphoma. 4th ed. Washington, DC: American Registry of Pathology;2017. p. 205–38.23. Pina-Oviedo S, Miranda RN, Lin P, Manning JT, Medeiros LJ. Follicular lymphoma with hyaline-vascular Castleman-like features: analysis of 6 cases and review of the literature. Hum Pathol. 2017; 68:136–46.
Article24. Kojima M, Yamanaka S, Yoshida T, et al. Histological variety of floral variant of follicular lymphoma. APMIS. 2006; 114:626–32.
Article25. Osborne BM, Butler JJ. Follicular lymphoma mimicking progressive transformation of germinal centers. Am J Clin Pathol. 1987; 88:264–9.
Article26. Coffing BN, Lim MS. Signet ring cell lymphoma in a patient with elevated CA-125. J Clin Oncol. 2011; 29:e416–8.
Article27. Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002; 99:3376–82.
Article28. Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011; 118:2976–84.
Article29. Vogelsberg A, Steinhilber J, Mankel B, et al. Genetic evolution of in situ follicular neoplasia to aggressive B-cell lymphoma of germinal center subtype. Haematologica. 2021; 106:2673–81.30. Schmidt J, Salaverria I, Haake A, et al. Increasing genomic and epigenomic complexity in the clonal evolution from in situ to manifest t(14;18)-positive follicular lymphoma. Leukemia. 2014; 28:1103–12.
Article31. Kosmidis P, Bonzheim I, Dufke C, et al. Next generation sequencing of the clonal IGH rearrangement detects ongoing mutations and interfollicular trafficking in in situ follicular neoplasia. PLoS One. 2017; 12:e0178503.
Article32. Adam P, Katzenberger T, Eifert M, et al. Presence of preserved reactive germinal centers in follicular lymphoma is a strong histopathologic indicator of limited disease stage. Am J Surg Pathol. 2005; 29:1661–4.
Article33. Marks E, Shi Y. Duodenal-type follicular lymphoma: a clinicopathologic review. Arch Pathol Lab Med. 2018; 142:542–7.
Article34. Hellmuth JC, Louissaint A Jr, Szczepanowski M, et al. Duodenaltype and nodal follicular lymphomas differ by their immune microenvironment rather than their mutation profiles. Blood. 2018; 132:1695–702.
Article35. Sato Y, Ichimura K, Tanaka T, et al. Duodenal follicular lymphomas share common characteristics with mucosa-associated lymphoid tissue lymphomas. J Clin Pathol. 2008; 61:377–81.
Article36. Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009; 113:1053–61.
Article37. Siddiqi IN, Friedman J, Barry-Holson KQ, et al. Characterization of a variant of t(14;18) negative nodal diffuse follicular lymphoma with CD23 expression, 1p36/TNFRSF14 abnormalities, and STAT6 mutations. Mod Pathol. 2016; 29:570–81.38. Finn LS, Viswanatha DS, Belasco JB, et al. Primary follicular lymphoma of the testis in childhood. Cancer. 1999; 85:1626–35.
Article39. Sovani V, Harvey C, Haynes AP, McMillan AK, Clark DM, O’Connor SR. Bone marrow trephine biopsy involvement by lymphoma: review of histopathological features in 511 specimens and correlation with diagnostic biopsy, aspirate and peripheral blood findings. J Clin Pathol. 2014; 67:389–95.
Article40. Torlakovic E, Torlakovic G, Brunning RD. Follicular pattern of bone marrow involvement by follicular lymphoma. Am J Clin Pathol. 2002; 118:780–6.
Article41. Mollejo M, Rodriguez-Pinilla MS, Montes-Moreno S, et al. Splenic follicular lymphoma: clinicopathologic characteristics of a series of 32 cases. Am J Surg Pathol. 2009; 33:730–8.42. Howard MT, Dufresne S, Swerdlow SH, Cook JR. Follicular lymphoma of the spleen: multiparameter analysis of 16 cases. Am J Clin Pathol. 2009; 131:656–62.43. Ferry JA, Leval L, Louissaint A, Harris NL. Follicular lymphoma. In : Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Martinez L, editors. Hematopathology. 2nd ed. Philadelphia: Elsevier;2017. p. 321–52.44. Menter T, Gasser A, Juskevicius D, Dirnhofer S, Tzankov A. Diagnostic uility of the germinal center-associated markers GCET1, HGAL, and LMO2 in hematolymphoid neoplasms. Appl Immunohistochem Mol Morphol. 2015; 23:491–8.45. Pittaluga S, Ayoubi TA, Wlodarska I, et al. BCL-6 expression in reactive lymphoid tissue and in B-cell non-Hodgkin’s lymphomas. J Pathol. 1996; 179:145–50.
Article46. Marafioti T, Copie-Bergman C, Calaminici M, et al. Another look at follicular lymphoma: immunophenotypic and molecular analyses identify distinct follicular lymphoma subgroups. Histopathology. 2013; 62:860–75.
Article47. Bosga-Bouwer AG, van Imhoff GW, Boonstra R, et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: t(14;18) and 3q27 are mutually exclusive. Blood. 2003; 101:1149–54.
Article48. Guo Y, Karube K, Kawano R, et al. Low-grade follicular lymphoma with t(14;18) presents a homogeneous disease entity otherwise the rest comprises minor groups of heterogeneous disease entities with Bcl2 amplification, Bcl6 translocation or other gene aberrances. Leukemia. 2005; 19:1058–63.
Article49. Nann D, Ramis-Zaldivar JE, Muller I, et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020; 4:5652–65.
Article50. Kendrick SL, Redd L, Muranyi A, et al. BCL2 antibodies targeted at different epitopes detect varying levels of protein expression and correlate with frequent gene amplification in diffuse large B-cell lymphoma. Hum Pathol. 2014; 45:2144–53.
Article51. Lai R, Weiss LM, Chang KL, Arber DA. Frequency of CD43 expression in non-Hodgkin lymphoma: a survey of 742 cases and further characterization of rare CD43+ follicular lymphomas. Am J Clin Pathol. 1999; 111:488–94.52. Tiesinga JJ, Wu CD, Inghirami G. CD5+ follicle center lymphoma. Immunophenotyping detects a unique subset of “floral” follicular lymphoma. Am J Clin Pathol. 2000; 114:912–21.53. Chang KC, Huang X, Medeiros LJ, Jones D. Germinal centre-like versus undifferentiated stromal immunophenotypes in follicular lymphoma. J Pathol. 2003; 201:404–12.
Article54. Wang SA, Wang L, Hochberg EP, Muzikansky A, Harris NL, Hasserjian RP. Low histologic grade follicular lymphoma with high proliferation index: morphologic and clinical features. Am J Surg Pathol. 2005; 29:1490–6.55. Hoglund M, Sehn L, Connors JM, et al. Identification of cytogenetic subgroups and karyotypic pathways of clonal evolution in follicular lymphomas. Genes Chromosomes Cancer. 2004; 39:195–204.56. Espinet B, Bellosillo B, Melero C, et al. FISH is better than BIOMED-2 PCR to detect IgH/BCL2 translocation in follicular lymphoma at diagnosis using paraffin-embedded tissue sections. Leuk Res. 2008; 32:737–42.
Article57. Leich E, Hoster E, Wartenberg M, et al. Similar clinical features in follicular lymphomas with and without breaks in the BCL2 locus. Leukemia. 2016; 30:854–60.
Article58. Chaudhary S, Brown N, Song JY, et al. Relative frequency and clinicopathologic characteristics of MYC-rearranged follicular lymphoma. Hum Pathol. 2021; 114:19–27.59. Bisso A, Sabo A, Amati B. MYC in germinal center-derived lymphomas: mechanisms and therapeutic opportunities. Immunol Rev. 2019; 288:178–97.60. Pasqualucci L, Khiabanian H, Fangazio M, et al. Genetics of follicular lymphoma transformation. Cell Rep. 2014; 6:130–40.
Article61. Huet S, Tesson B, Jais JP, et al. A gene-expression profiling score for prediction of outcome in patients with follicular lymphoma: a retrospective training and validation analysis in three international cohorts. Lancet Oncol. 2018; 19:549–61.
Article62. Elenitoba-Johnson KS, Gascoyne RD, Lim MS, Chhanabai M, Jaffe ES, Raffeld M. Homozygous deletions at chromosome 9p21 involving p16 and p15 are associated with histologic progression in follicle center lymphoma. Blood. 1998; 91:4677–85.
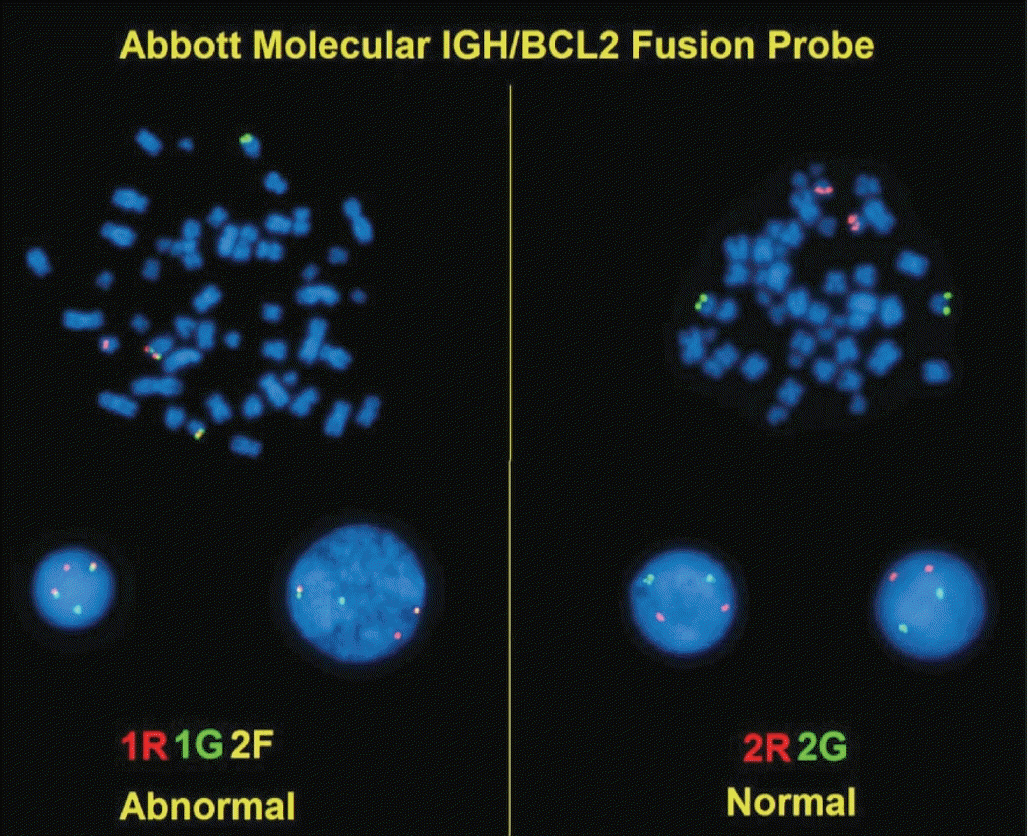
Article63. Bouska A, McKeithan TW, Deffenbacher KE, et al. Genome-wide copy-number analyses reveal genomic abnormalities involved in transformation of follicular lymphoma. Blood. 2014; 123:1681–90.
Article64. Evans PA, Pott C, Groenen PJ, et al. Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets. Report of the BIOMED-2 Concerted Action BHM4-CT98-3936. Leukemia. 2007; 21:207–14.
Article65. Bagg A, Braziel RM, Arber DA, Bijwaard KE, Chu AY. Immunoglobulin heavy chain gene analysis in lymphomas: a multi-center study demonstrating the heterogeneity of performance of polymerase chain reaction assays. J Mol Diagn. 2002; 4:81–9.66. Weiss LM, O’Malley D. Benign lymphadenopathies. Mod Pathol. 2013; 26 Suppl 1:S88–96.
Article67. Mantei K, Wood BL. Flow cytometric evaluation of CD38 expression assists in distinguishing follicular hyperplasia from follicular lymphoma. Cytometry B Clin Cytom. 2009; 76:315–20.
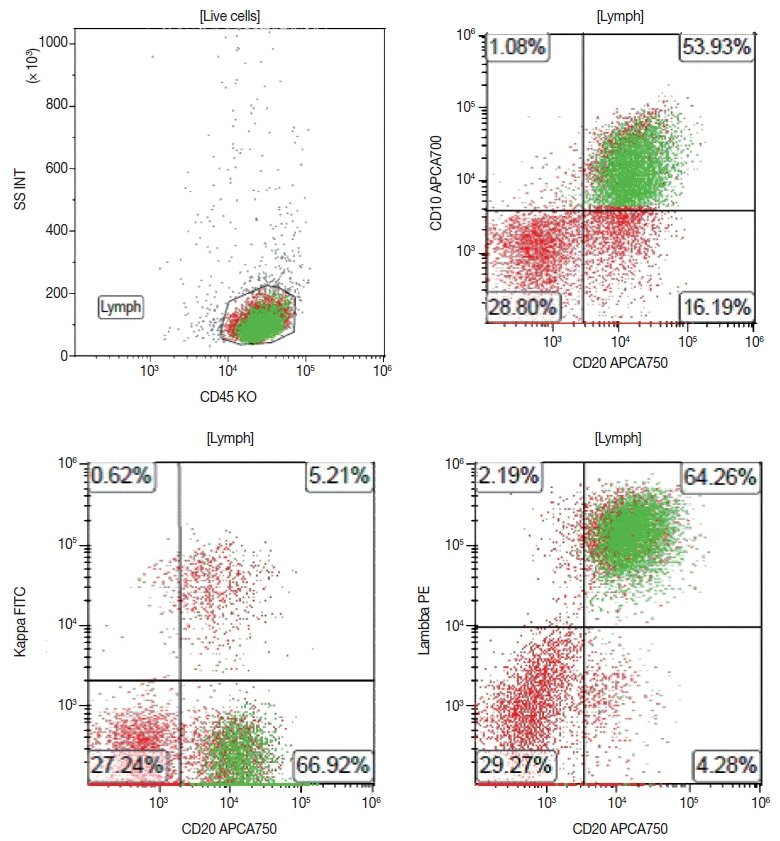
Article68. Seegmiller AC, Hsi ED, Craig FE. The current role of clinical flow cytometry in the evaluation of mature B-cell neoplasms. Cytometry B Clin Cytom. 2019; 96:20–9.
Article69. Khanlari M, Wang SA, Tang G, Saluja K, Medeiros LJ, Thakral B. CD45-negative follicular lymphoma: a rare diagnostic pitfall of a common entity. Cytometry B Clin Cytom. 2021; 100:406–8.70. Younes S, Rojansky RB, Menke JR, Gratzinger D, Natkunam Y. Pitfalls in the diagnosis of nodular lymphocyte predominant Hodgkin lymphoma: variant patterns, borderlines and mimics. Cancers (Basel). 2021; 13:3021.
Article71. Poveda J, Cassidy DP, Zhou Y, et al. Expression of germinal center cell markers by extranodal marginal zone lymphomas of MALT type within colonized follicles, a diagnostic pitfall with follicular lymphoma. Leuk Lymphoma. 2021; 62:1116–22.
Article72. Wang Z, Cook JR. IRTA1 and MNDA expression in marginal zone lymphoma: utility in differential diagnosis and implications for classification. Am J Clin Pathol. 2019; 151:337–43.73. Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol. 2019; 184:660–3.
Article74. Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015; 33:2516–22.
Article75. Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled analysis of French and US cohorts. J Clin Oncol. 2019; 37:144–52.
Article76. Kridel R, Chan FC, Mottok A, et al. Histological transformation and progression in follicular lymphoma: a clonal evolution study. PLoS Med. 2016; 13:e1002197.
Article77. Lee AY, Connors JM, Klimo P, O'Reilly SE, Gascoyne RD. Late relapse in patients with diffuse large-cell lymphoma treated with MACOP-B. J Clin Oncol. 1997; 15:1745–53.
Article78. Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004; 104:1258–65.
Article79. Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009; 27:4555–62.
Article80. Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015; 16:1111–22.
Article81. Wahlin BE, Yri OE, Kimby E, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012; 156:225–33.
Article82. Ross CW, Ouillette PD, Saddler CM, Shedden KA, Malek SN. Comprehensive analysis of copy number and allele status identifies multiple chromosome defects underlying follicular lymphoma pathogenesis. Clin Cancer Res. 2007; 13:4777–85.
Article83. Khanlari M, Wang SA, Fowler NH, et al. Concurrent TP53 mutation and deletion in refractory low-grade follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2021; 21:e626–9.
Article84. Ollila TA, Olszewski AJ. Chemotherapy-free management of follicular and marginal zone lymphoma. Cancer Manag Res. 2021; 13:3935–52.
Article85. Schuster SJ. Bispecific antibodies for the treatment of lymphomas: promises and challenges. Hematol Oncol. 2021; 39 Suppl 1:113–6.
Article86. Mondello P, Steiner N, Willenbacher W, et al. Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with follicular lymphoma grade 3A: evidence from a multicenter, retrospective study. Oncologist. 2018; 23:454–60.
Article87. Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017; 28:2169–78.
Article88. Pagel JM, Burke JM, Leslie LA. Refining the management of relapsed or refractory follicular lymphoma: case scenarios. Clin Adv Hematol Oncol. 2020; 18 Suppl 20:20–1.89. Pongas G, Cheson B. Recent advances in the management of patients with relapsed/refractory follicular lymphoma. Blood Lymphat Cancer. 2021; 11:55–66.
Article90. Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018; 19:649–59.91. Evens AM, Balasubramanian S, Vose JM, et al. A phase I/II multicenter, open-label study of the oral histone deacetylase inhibitor abexinostat in relapsed/refractory lymphoma. Clin Cancer Res. 2016; 22:1059–66.
Article92. Ribrag V, Kim WS, Bouabdallah R, et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica. 2017; 102:903–9.
Article93. Sawalha Y, Maddocks K. Profile of polatuzumab vedotin in the treatment of patients with relapsed/refractory non-Hodgkin Lymphoma: a brief report on the emerging clinical data. Onco Targets Ther. 2020; 13:5123–33.94. Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018; 379:1711–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dermatomyositis Associated with Follicular Lymphoma
- Composite follicular lymphoma and classic Hodgkin lymphoma
- Smad1 Expression in Follicular Lymphoma
- A Rare Case of Primary Duodenal Follicular Lymphoma
- Detection of bcl-2/IgH Gene Rearrangement and Expression of c-myc and p53 Oncoprotein in B-cell Lymphoma