J Korean Acad Oral Health.
2021 Dec;45(4):204-209. 10.11149/jkaoh.2021.45.4.204.
Antibacterial activity of phytoncide on oral biofilm
- Affiliations
-
- 1Department of Oral Health, College of Health Science, Dankook University, Cheonan, Korea
- 2Department of Biomedical Science and Engineering, Konkuk University, Chungju, Korea
- 3Department of Pediatric Dentistry, College of Dentistry, Dankook University, Cheonan, Korea
- 4Department of Preventive Dentistry, College of Dentistry, Dankook University, Cheonan, Korea
- KMID: 2524197
- DOI: http://doi.org/10.11149/jkaoh.2021.45.4.204
Abstract
Objectives
This study aimed to evaluate the antibacterial activity of phytoncide on oral biofilm.
Methods
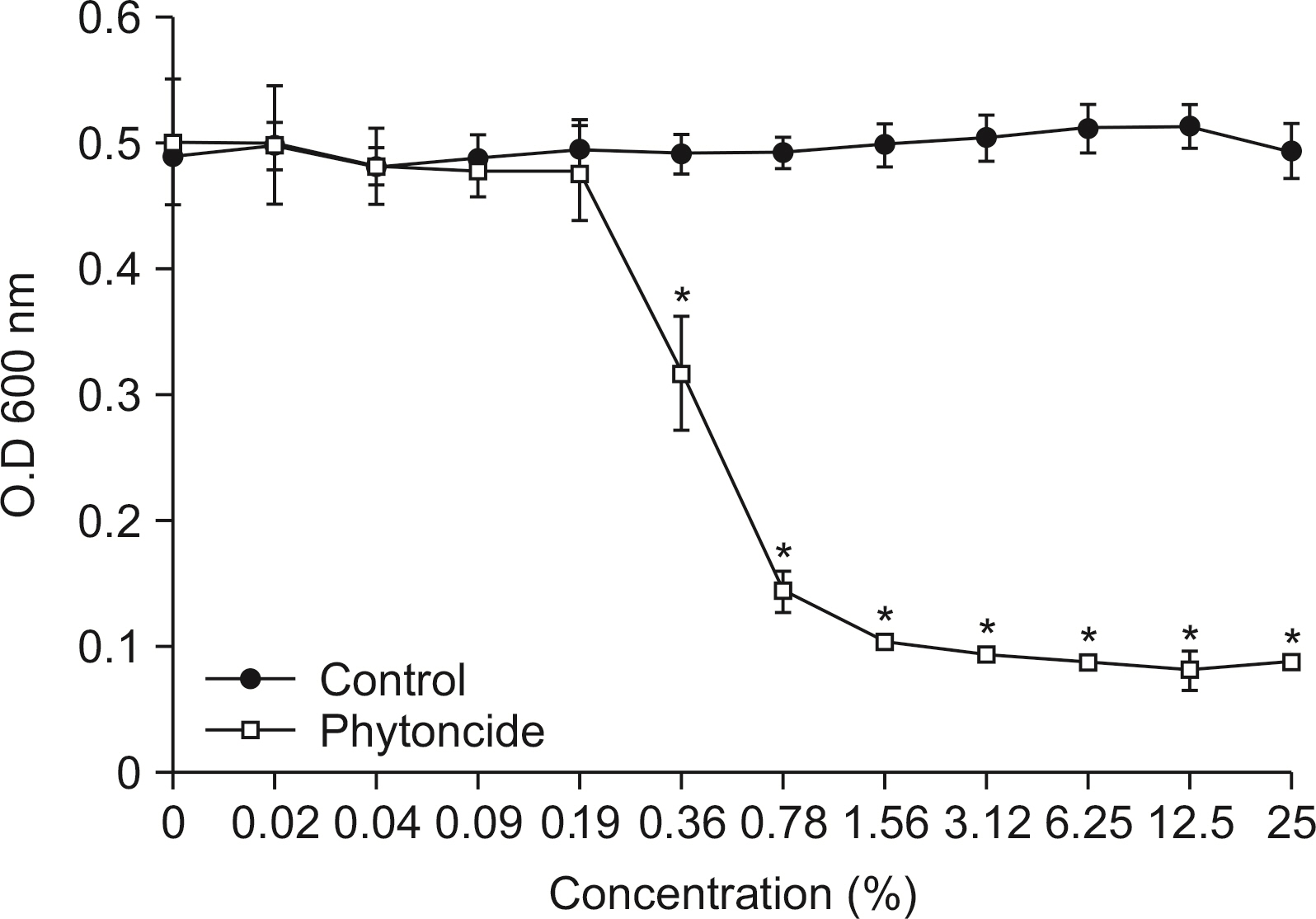
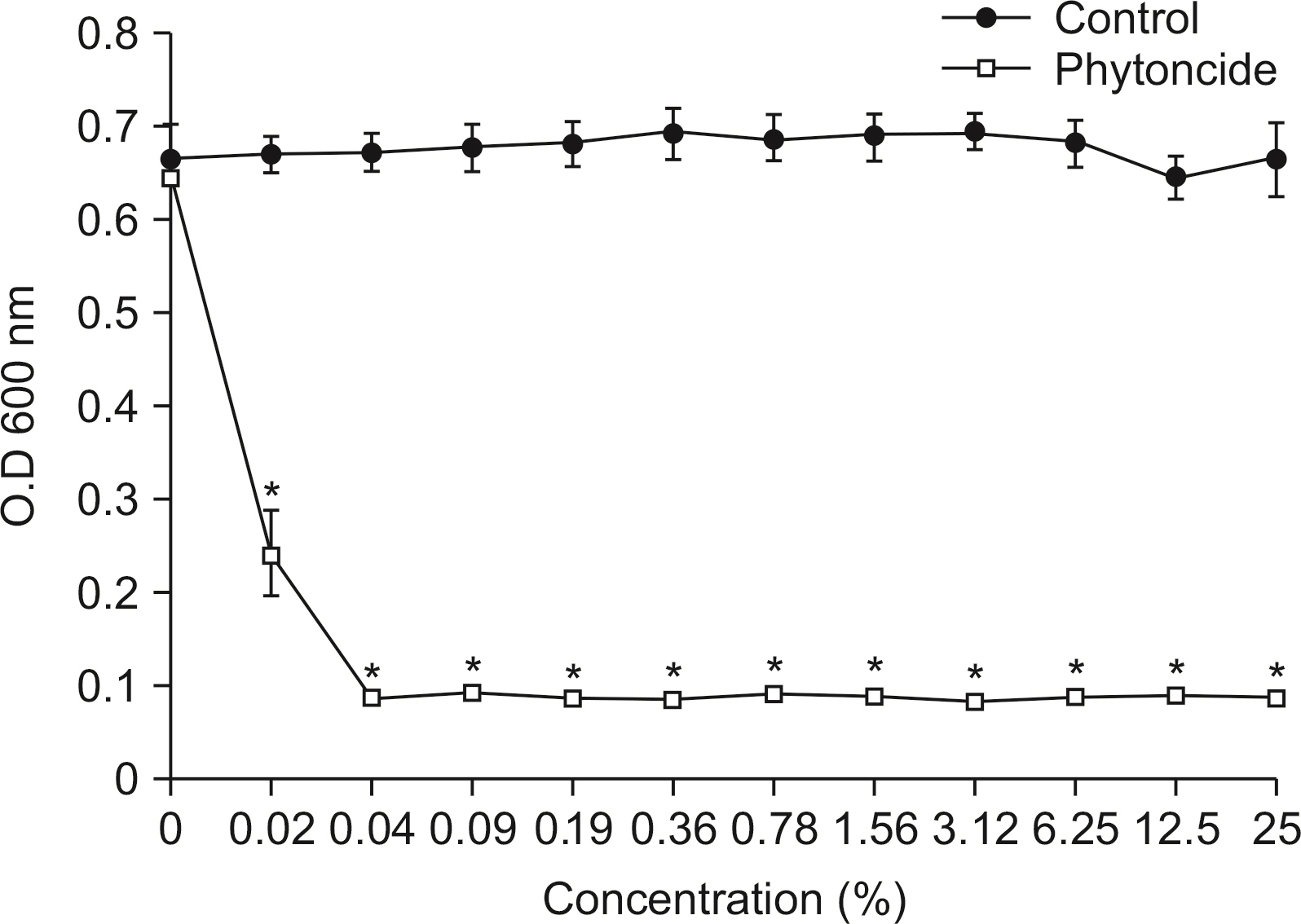
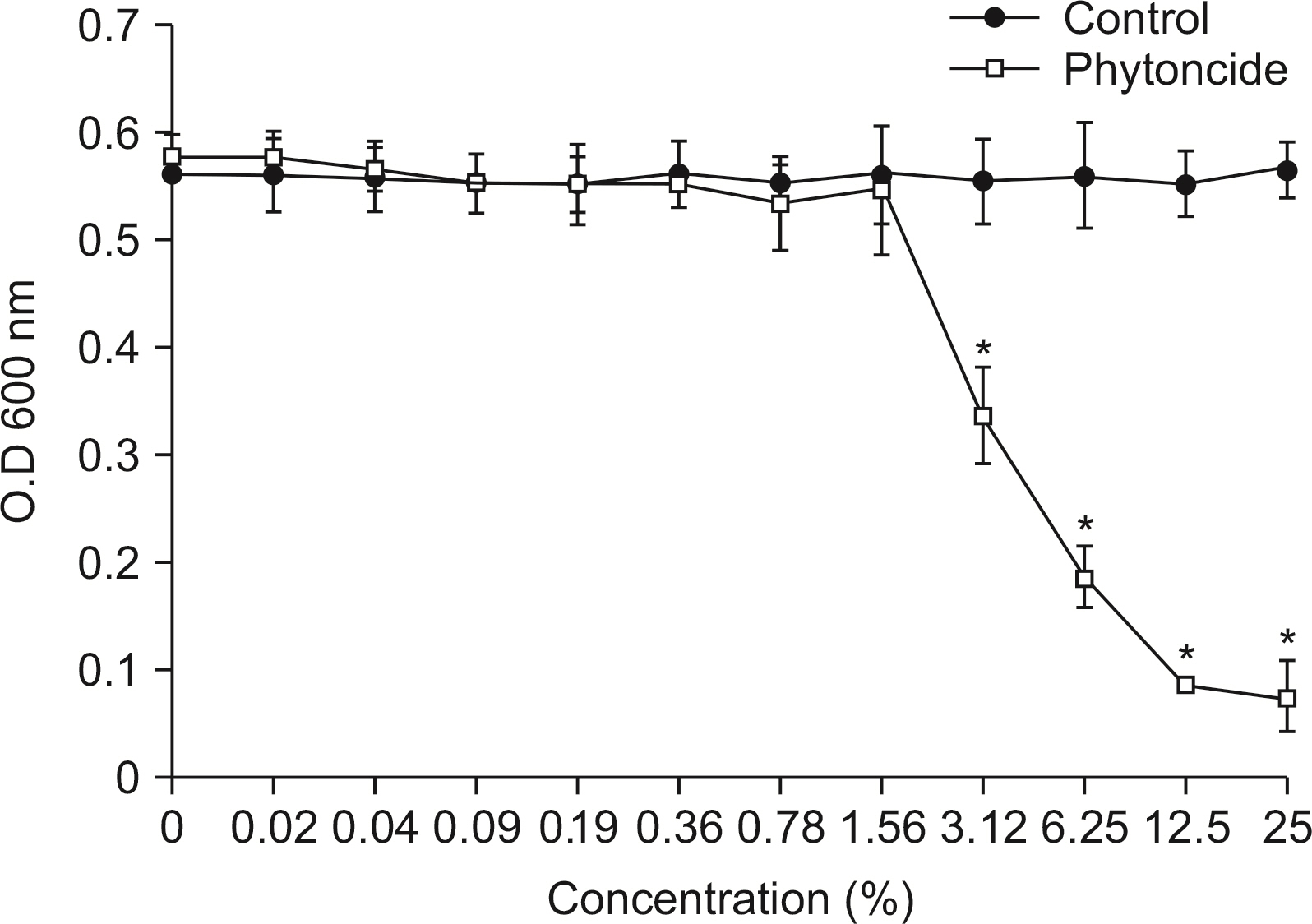
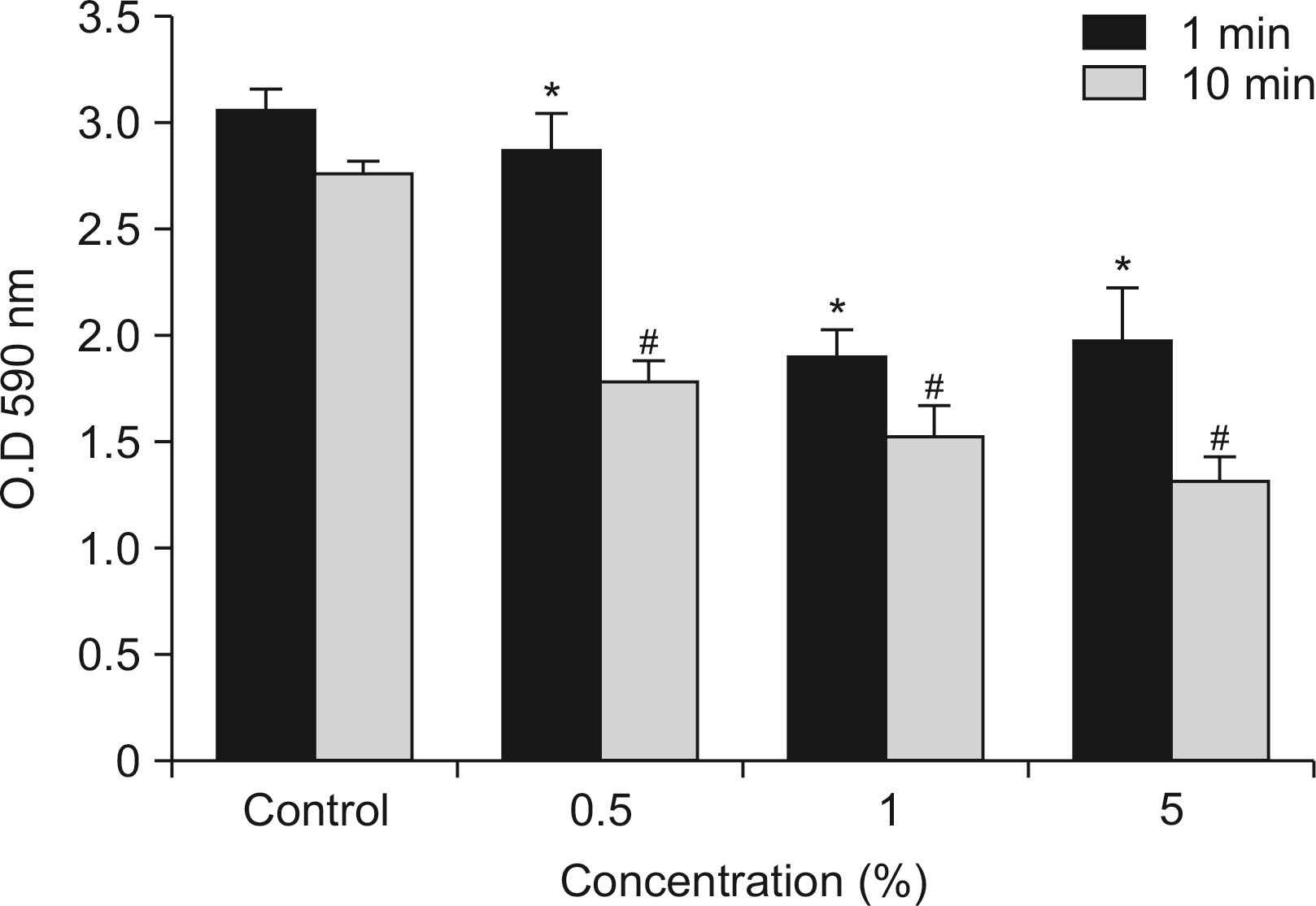
Antibacterial activity of phytoncide was examined on the bacteria Streptococcus mutans, Aggregatibacter actinomycetemcomitans, Enterococcus faecalis, Porphyromonas gingivalis, and oral biofilm. After mixing the phytoncide, S. mutans, A. actinomycetemcomitans, E. faecalis, and P. gingivalis with a culture alone turbid and mixed, and they were then incubated at 37°C under anaerobic conditions and cultured. Following incubation, a microplate reader was used to measure the absorbance and observe the amount of bacteria. In addition, non-polarized saliva containing bacteria was grown for 72 h under anaerobic conditions for the determination of biofilm biomass and bacterial numbers. Various concentrations of phytoncide were added to the saliva biofilm. Statistical significance tests were conducted using the Mann-Whitney test and SPSS 24.0.
Results
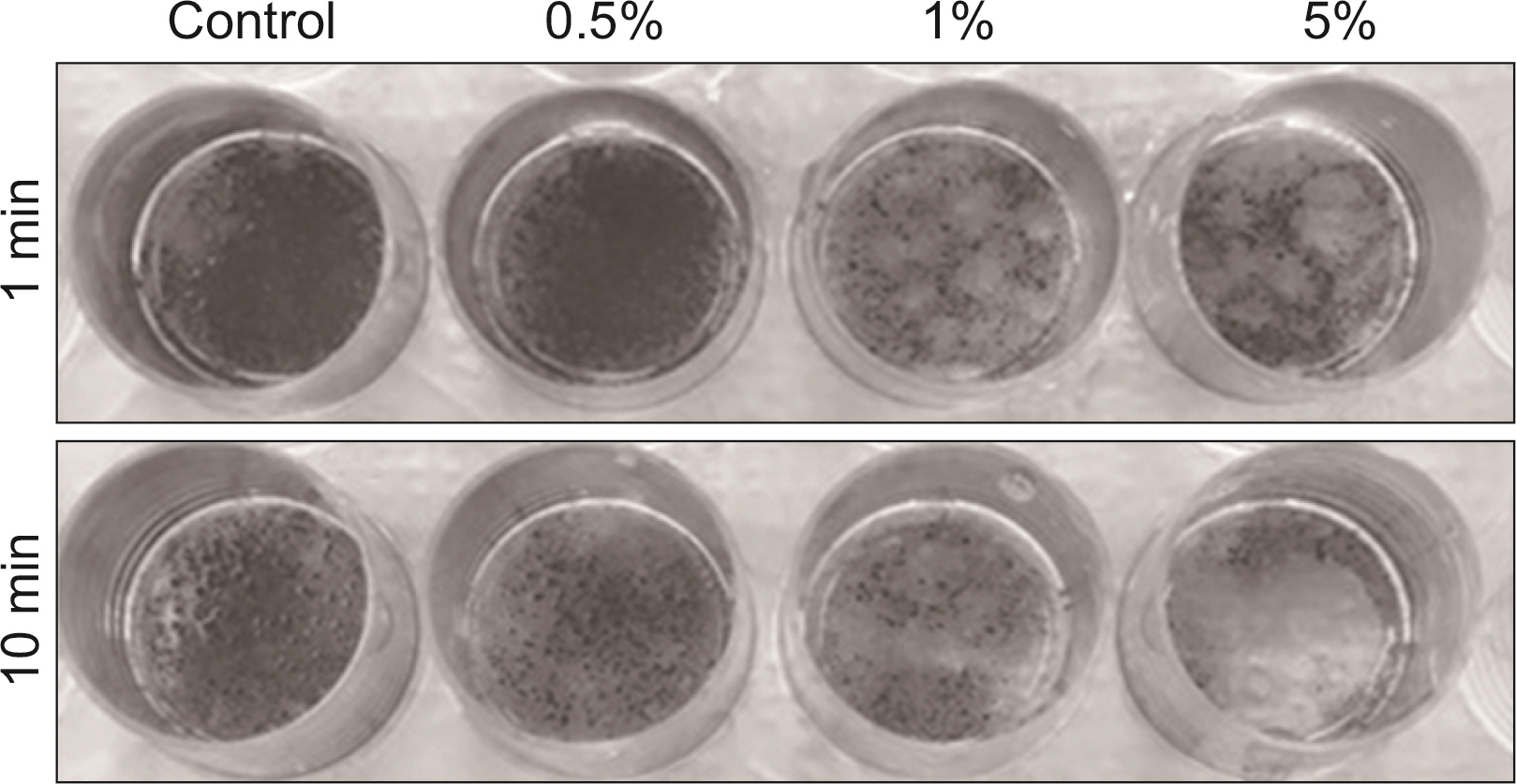
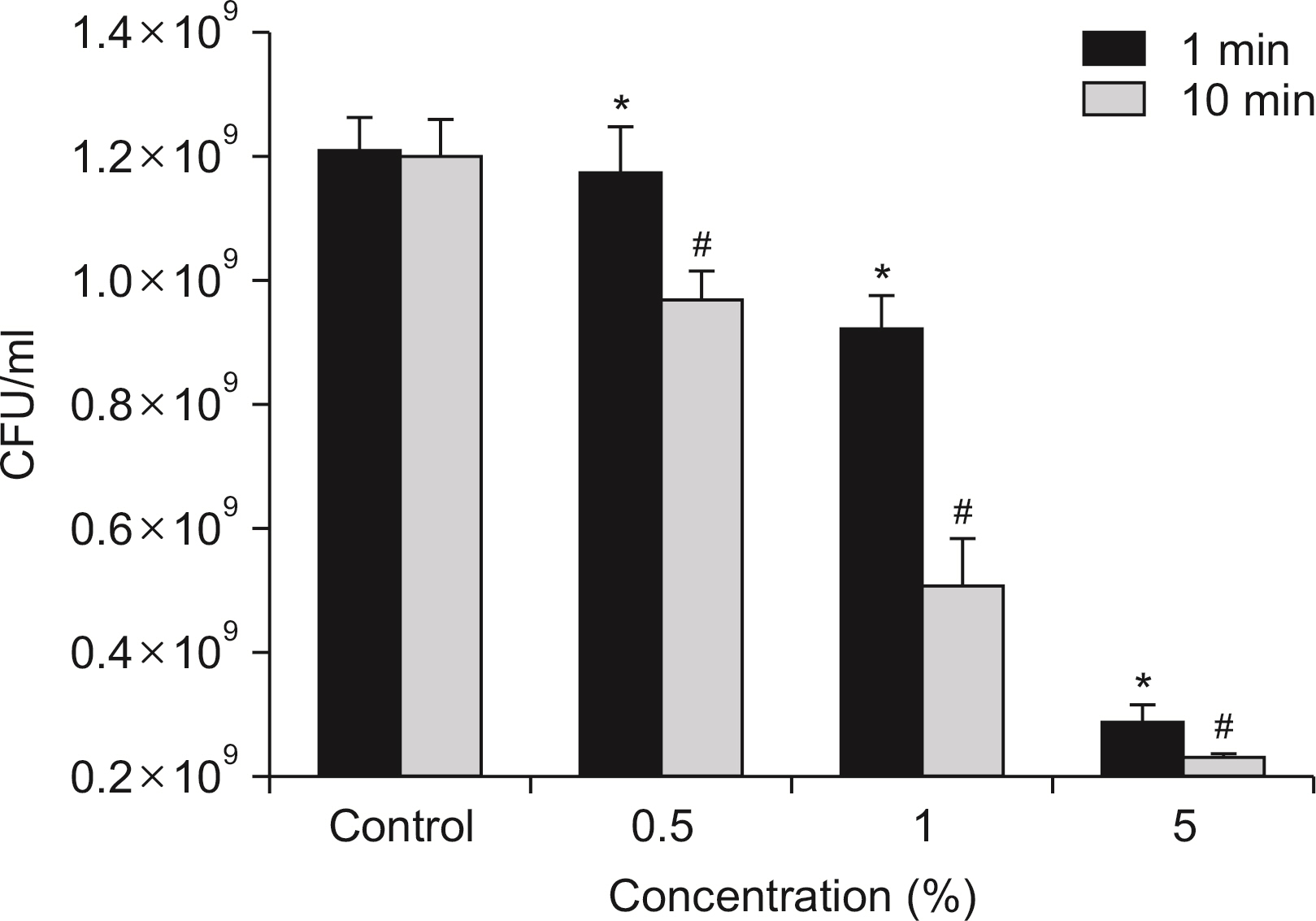
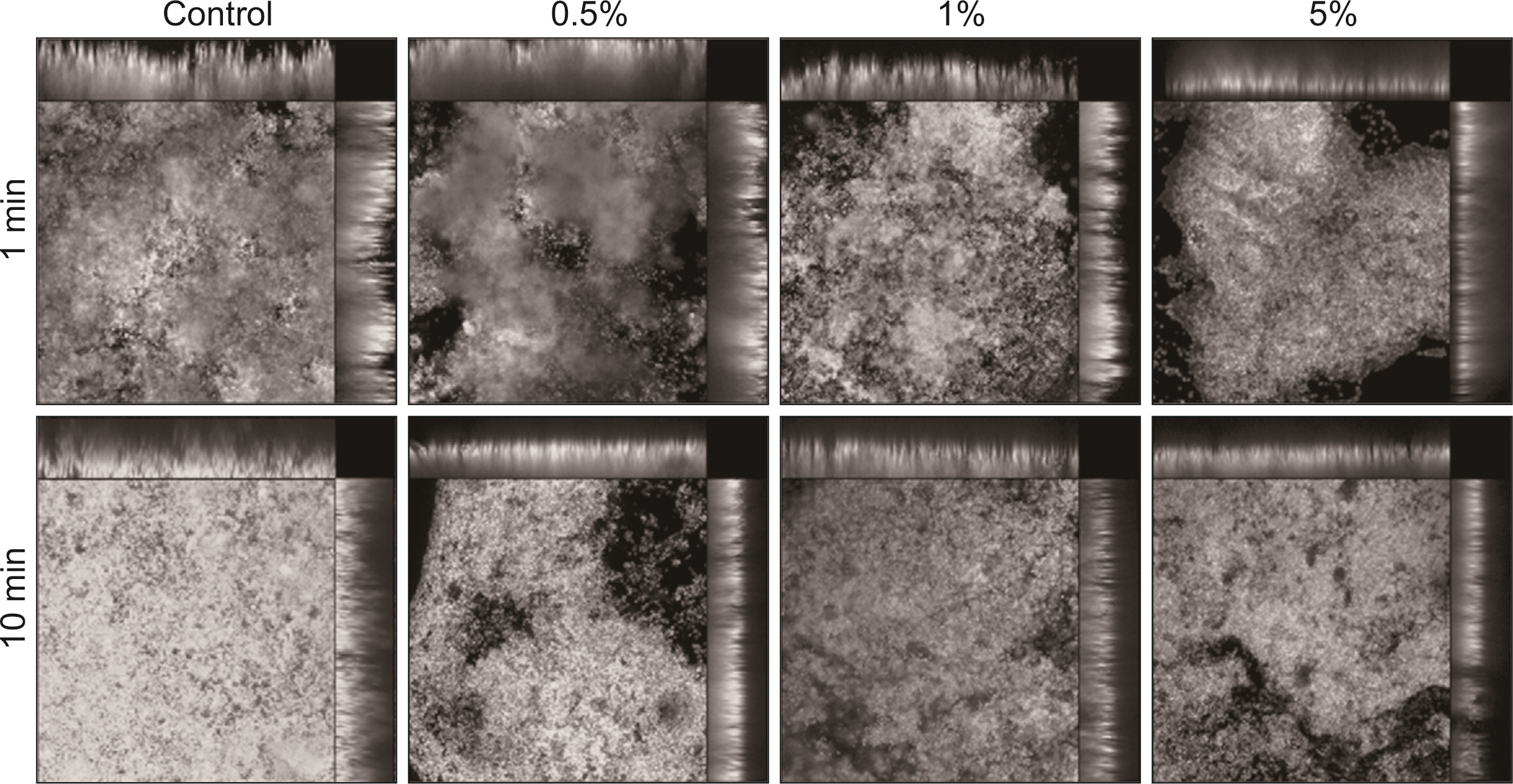
S. mutans, A. actinomycetemcomitans, E. faecalis, P. gingivalis, and antibacterial effects were shown as phytoncide concentrations increased in oral biofilm. Observing the growth of bacteria using phase difference microscopes showed that the number of bacteria decreases as the phytoncide concentration increased. Also, the formation of biofilm in the saliva decreased compared to the control group.
Conclusions
Based on the experimental results of phytoncide on oral biofilms causing oral cavity formation in the saliva decreased compared to that in the control group. Phytoncide showed antibiosis against oral biofilms when it remained inside the mouth for above certain concentrations. Accordingly, using phytoncide as a clinical method for preventing oral disease is deemed to be effective.
Keyword
Figure
Reference
-
References
1. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005; Nov. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 43:5721–5732. DOI: 10.1128/JCM.43.11.5721-5732.2005. PMID: 16272510. PMCID: PMC1287824.
Article2. Kolenbrander PE1, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ Jr. 2002; Sep. Communication among oral bacteria. Microbiol Mol Biol Rev. 66:486–505. DOI: 10.1128/MMBR.66.3.486-505.2002. PMID: 12209001. PMCID: PMC120797.
Article3. Kang HY, Lee SS, Choi IK. 1993; The Antifungal Activity of Coniferous Needle Oil. J of Kor forestry energy. 13:71–77.4. Whittaker RH, Feeny PP. 1997; Allelochemics: chemical interactions between spices. Science. 171:757–770. DOI: 10.1126/science.171.3973.757. PMID: 5541160.5. Welsh C. 1997; Touch with oils-a pertinent part of holistic care. Am J Hosp Palliat Care. 14:42–4. DOI: 10.1177/104990919701400114. PMID: 9069764.6. Lis-Balchin M. 1977; Essential oils and 'aromatherapy': their modern role in healing. J R Soc Health. 117:324–329. DOI: 10.1177/146642409711700511. PMID: 9519666.
Article7. Chung JY, Choo JH, Lee MH, Hwang JK. 2006; Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 13:261–266. DOI: 10.1016/j.phymed.2004.04.007. PMID: 16492529.
Article8. Scheie AA, Petersen FC. 2004; The Biofilm Concept: Consequences for Future Prophylaxis of Oral Diseases? Crit Rev Oral Biol Med. 15:4–12. DOI: 10.1177/154411130401500102. PMID: 14761896.
Article9. Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. 2002; Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 40:1001–1009. DOI: 10.1128/JCM.40.3.1001-1009.2002. PMID: 11880430. PMCID: PMC120252.
Article10. Socransky SS, Haffajee AD. 2002; Dental biofilms: difficult therapeutic targets. Periodontol. 28:12–55. DOI: 10.1034/j.1600-0757.2002.280102.x. PMID: 12013340.
Article11. Jorgensen JH. 1993; Antimicrobial susceptibility testing of bacteria that grow aerobically. Infect Dis Clin North Am. 7:393–409. DOI: 10.1016/S0891-5520(20)30528-6. PMID: 8345175.
Article12. Rosan B, Lamont RJ. 2000; Dental plaque formation. Microbes Infect. 2:1599–1607. DOI: 10.1016/S1286-4579(00)01316-2. PMID: 11113379.
Article13. Minah GE, Loesche WJ. 1977; Sucrose metabolism by prominent members of the flora isolated from cariogenic and non-cariogenic dental plaques. Infect Immun. 17:55–61. DOI: 10.1128/iai.17.1.55-61.1977. PMID: 407163. PMCID: PMC421080.
Article14. Rice LB. 2006; Antimicrobial resistance in gram-positive bacteria. Am J Med. 119Suppl1:62–70. DOI: 10.1016/j.amjmed.2006.03.012. PMID: 16735146.
Article15. Rice EL. 1984. Allelopathy. 2nd ed. Acamedic Press Inc;New York: p. 79–110.16. Lee HO, Baek SH, Han DM. 2001; Antimicrobial Effects of Chamaecyparis obtusa Essential Oil. Korean J of Microbiology and Biotechnology. 29:253–257.17. Park MJ, Jung EB, Choi IK, Lee SM, Kwak KS, Jang JW. 2005; Investigation of Active Antifungal Compounds of Essential Oil from Chamaecyparis obtusa Against Dermatophytes, Microsporum canis and Trichophyton Mentagrophytes. J Korean Wood Science and Tech. 33:72–78.18. Kim SK, Shin MK, EO KS, LEE JY, HONG JP, Jung YH. 2007; Effect of Phytoncide on Porphyromonas gingivalis. J Oral Med Pain. 32:137–150.19. Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. 2004; A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol. 19:61–64. DOI: 10.1046/j.0902-0055.2003.00111.x. PMID: 14678476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibacterial Efficacy of Dental Sealant Containing Phytoncide
- Galla chinensis extracts and calcium induce remineralization and antibacterial effects of enamel in a Streptococcus mutans biofilm model
- Inhibitory Effect of Pentose on Biofilm Formation by Oral Bacteria
- Inhibition of biofilm formation of periodontal pathogens by D-Arabinose
- Antibacterial effect of genistein against periodontal pathogens