Neonatal Med.
2021 Nov;28(4):161-166. 10.5385/nm.2021.28.4.161.
A Case of Neonatal Thyrotoxicosis with Cardiac Insufficiency
- Affiliations
-
- 1Department of Pediatrics, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2522854
- DOI: http://doi.org/10.5385/nm.2021.28.4.161
Abstract
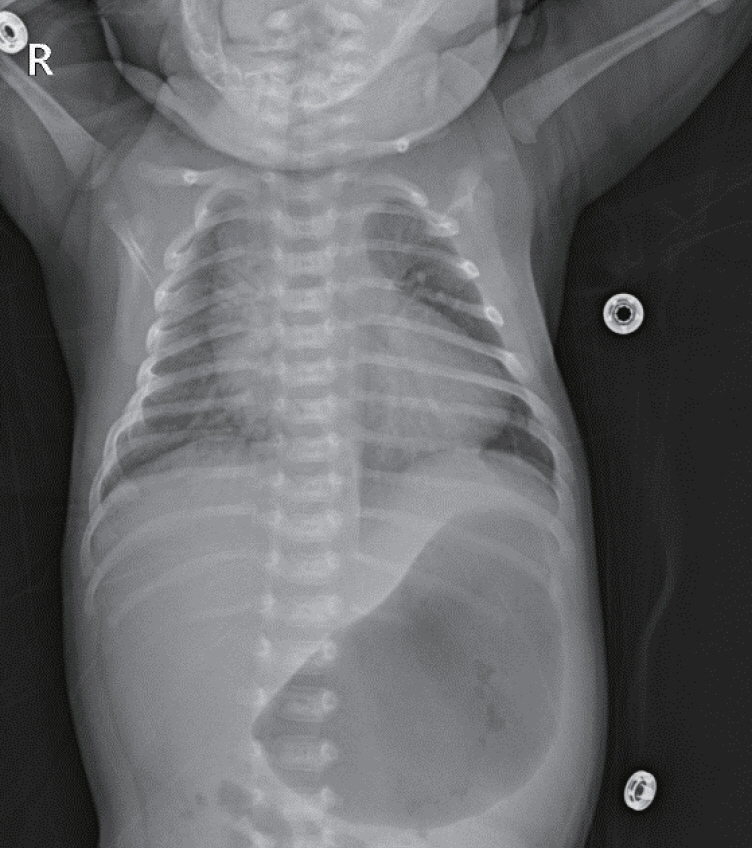
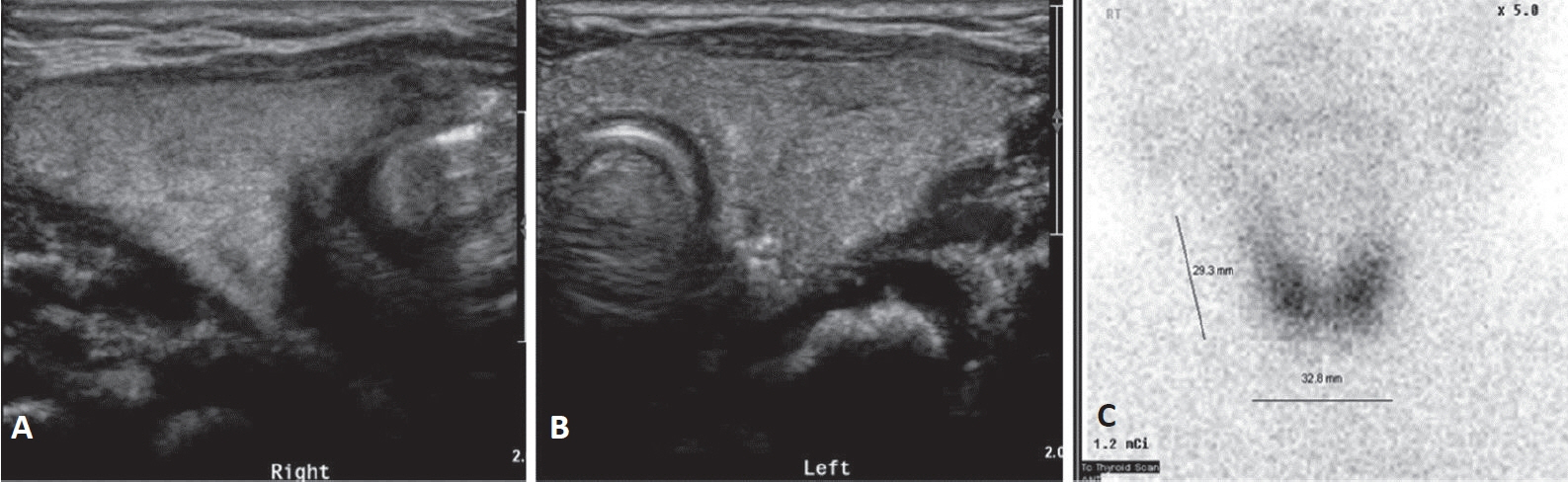
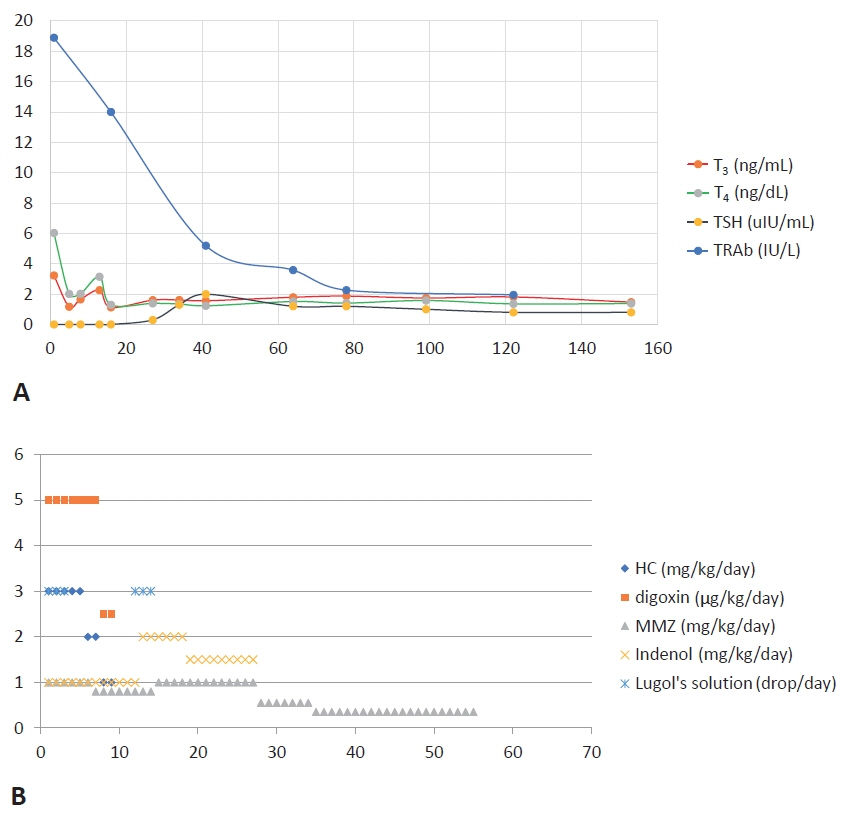
- Neonatal thyrotoxicosis is rare and most of the cases are secondary to maternal Graves’ disease. It is usually transient, but can be associated with significant morbidity and mortality if not recognized promptly and treated adequately. Neonates born to mothers treated with antithyroid drugs or those who receive maternal thyroid blocking antibodies may exhibit normal thyroid function or even hypothyroidism at birth. Since there may not be any obvious symptoms of hyperthyroidism at birth, it may be overlooked. Therefore, such neonates should be evaluated properly and monitored regularly to prevent serious complications of hyperthyroidism. We report a case of a 21-day-old male infant who developed thyrotoxicosis with dyspnea, irritability, tachycardia, and cardiac insufficiency. He was born to a mother who was treated for Graves’ disease with antithyroid drugs during pregnancy. We have also discussed the importance of careful examination and monitoring to prevent the development of clinical hyperthyroidism.
Figure
Reference
-
1. Samuels SL, Namoc SM, Bauer AJ. Neonatal thyrotoxicosis. Clin Perinatol. 2018; 45:31–40.2. Segni M. Neonatal hyperthyroidism. In : Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. Endotext. South Dartmouth: MDText.com;2000. https://www.ncbi.nlm.nih.gov/books/NBK279019.3. van Dijk MM, Smits IH, Fliers E, Bisschop PH. Maternal thyrotropin receptor antibody concentration and the risk of fetal and neonatal thyrotoxicosis: a systematic review. Thyroid. 2018; 28:257–64.4. Besancon A, Beltrand J, Le Gac I, Luton D, Polak M. Management of neonates born to women with Graves’ disease: a cohort study. Eur J Endocrinol. 2014; 170:855–62.5. Leger J. Management of fetal and neonatal Graves’ disease. Horm Res Paediatr. 2017; 87:1–6.6. Volpe R, Ehrlich R, Steiner G, Row VV. Graves’ disease in pregnancy years after hypothyroidism with recurrent passive-transfer neonatal Graves’ disease in offspring: therapeutic considerations. Am J Med. 1984; 77:572–8.7. Perry RJ, Hollman AS, Wood AM, Donaldson MD. Ultrasound of the thyroid gland in the newborn: normative data. Arch Dis Child Fetal Neonatal Ed. 2002; 87:F209–11.8. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27:315–89.9. Kiefer FW, Klebermass-Schrehof K, Steiner M, Worda C, Kasprian G, Diana T, et al. Fetal/neonatal thyrotoxicosis in a newborn from a hypothyroid woman with Hashimoto thyroiditis. J Clin Endocrinol Metab. 2017; 102:6–9.10. Abeillon-du Payrat J, Chikh K, Bossard N, Bretones P, Gaucherand P, Claris O, et al. Predictive value of maternal secondgeneration thyroid-binding inhibitory immunoglobulin assay for neonatal autoimmune hyperthyroidism. Eur J Endocrinol. 2014; 171:451–60.11. van der Kaay DC, Wasserman JD, Palmert MR. Management of neonates born to mothers with Graves’ disease. Pediatrics. 2016; 137:e20151878.12. Zakarija M, McKenzie JM, Hoffman WH. Prediction and therapy of intrauterine and late-onset neonatal hyperthyroidism. J Clin Endocrinol Metab. 1986; 62:368–71.13. Goh J, Cho HS, Oh PS, Cha JK, Kim JW, Park CY, et al. A case of neonatal Graveses disease. J Korean Soc Pediatr Endocrinol. 1999; 4:104–8.14. Oh SM, Kim HS, Sung TJ, Yang S. A case of neonatal thyrotoxicosis detected by neonatal tachycardia. Korean J Perinatol. 2008; 19:80–3.15. Banige M, Polak M, Luton D; Research Group for Perinatal Dysthyroidism (RGPD) Study Group. Prediction of neonatal hyperthyroidism. J Pediatr. 2018; 197:249–54.16. Kempers MJ, van Trotsenburg AS, van Rijn RR, Smets AM, Smit BJ, de Vijlder JJ, et al. Loss of integrity of thyroid morphology and function in children born to mothers with inadequately treated Graves’ disease. J Clin Endocrinol Metab. 2007; 92:2984–91.17. Kempers MJ, van Tijn DA, van Trotsenburg AS, de Vijlder JJ, Wiedijk BM, Vulsma T. Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab. 2003; 88:5851–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of neonatal thyrotoxicosis detected by neonatal tachycardia
- A Patient with Primary Amyloidosis Misrecognized as Thyrotoxicosis-induced Heart Failure
- A Case of Thyrotoxic Hypokalemic Periodic Paralysis Presenting as Cardiac Arrest
- A Case of Transient Thyrotoxicosis Associated with Acute Hemorrhagic Necrosis of a Thyroid Nodule
- Improvement of Severe Tricuspid Regurgitation with Right Heart Failure Associated with Thyrotoxicosis due to Graves' Disease